Class 12 : Physics (English) – Chapter 12: Atoms
EXPLANATION & SUMMARY
🟢 Rutherford’s Scattering Experiment (With Derivation)

Setup: A thin gold foil bombarded with α-particles.
Observations:
Most α-particles passed straight through → atom mostly empty.
Some deflected at small angles → nucleus repels positive charge.
A very few deflected backward → nucleus very dense, tiny.
Derivation of Scattering Formula (outline in NCERT):
Force between α-particle and nucleus:
F = (1/4πε₀) (Z₁Z₂e²/r²)
Closest approach distance (r₀): when KE = PE.
(1/2)mv² = (1/4πε₀)(Z₁Z₂e²/r₀)
r₀ = (1/4πε₀)(Z₁Z₂e²) / (1/2)mv²
For gold (Z=79) and α-particle (Z=2), distance of closest approach calculated in experiments is ~3 × 10⁻¹⁴ m, showing nuclear size is extremely small compared to atom (~10⁻¹⁰ m).
🔴 Atomic Spectra
Formula:
1/λ = RZ²(1/n₁² – 1/n₂²)
Example: For hydrogen (Z=1), Balmer line for n₁=2, n₂=3:
1/λ = R(1/2² – 1/3²) = R(1/4 – 1/9) = R(5/36).
Thus λ ≈ 656 nm, a visible red line.
🟡 Bohr’s Model with Step Derivation
Angular Momentum Quantization:
L = mvr = nh/2π
Centripetal Force:
mv²/r = (1/4πε₀)(Ze²/r²)
Substituting v from angular momentum:
m(vr) = nh/2π → v = nh/(2πmr).
Solve to get:
Radius of nth orbit:
rn = (ε₀h²n²)/(πme²Z)
Velocity of nth orbit:
vn = e²Z/(2ε₀h)(1/n)
Total Energy:
En = –(13.6 eV × Z²)/n²
Thus for hydrogen (Z=1, n=1), E₁ = –13.6 eV.
Ionization energy: energy required to remove electron from ground state = 13.6 eV.
🔵 de Broglie Hypothesis

Electron wave: λ = h/p.
Stable orbit → circumference = integer multiple of wavelength:
2πrn = nλ.
Thus Bohr’s quantization has wave interpretation.
🟢 Quantum Mechanical Model
Electrons as standing waves described by Schrödinger’s ψ.
|ψ|² gives probability density.
Orbits replaced by orbitals (s, p, d, f shapes in higher study).
🔴 Franck–Hertz Experiment
Electrons accelerated through mercury vapor. Current decreased at ~4.9 V → electrons lose energy equal to excitation of mercury. Proved quantized energy levels.
🟡 Applications
Explains atomic emission/absorption spectra.
Basis for astrophysical spectroscopy.
Foundation for modern quantum mechanics.
Used in developing semiconductors and nuclear science.
✨ Summary (~300 words, Concise & Revision-Friendly)
Rutherford’s α-scattering experiment proved atoms have a dense central nucleus, with electrons outside and most of the atom empty. But classical theory predicted such atoms should collapse due to radiation.
Spectroscopy revealed discrete lines, notably hydrogen series (Lyman in UV, Balmer in visible, Paschen/Brackett/Pfund in IR). Rydberg’s formula expressed these lines mathematically.
Bohr solved the stability issue by proposing quantized orbits. His postulates included angular momentum quantization (nh/2π) and energy exchange only during transitions. Derivations yielded expressions for orbit radii, electron velocity, and energy levels. He explained hydrogen’s spectrum precisely, predicting ionization energy (13.6 eV).
de Broglie interpreted Bohr’s quantization using matter waves: only those orbits that support standing waves are stable.
Quantum mechanics replaced exact orbits with orbitals. Schrödinger’s equation provided probability distributions for electrons.
Franck–Hertz experiment gave strong evidence for discrete levels, confirming theoretical ideas.
Applications include spectroscopy, astrophysics, electronics, and quantum science.
📝 Quick Recap
🔵 Rutherford: nucleus discovered; distance of closest approach formula.
🟢 Spectra: Hydrogen lines explained by Rydberg formula.
🔴 Bohr: quantized orbits; En = –13.6 eV/n².
🟡 de Broglie: wave interpretation of quantization.
✔️ Schrödinger: orbitals, probability clouds.
💡 Franck–Hertz: experimental proof.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
Question 12.1
Choose the correct alternative from the clues given at the end of each statement:
(a) The size of the atom in Thomson’s model is ……… the atomic size in Rutherford’s model. (much greater than / no different from / much less than)
(b) In the ground state of ……… electrons are in stable equilibrium, while in ……… electrons always experience a net force. (Thomson’s model / Rutherford’s model)
(c) A classical atom based on ……… is doomed to collapse. (Thomson’s model / Rutherford’s model)
(d) An atom has a nearly continuous mass distribution in a ……… but has a highly non-uniform mass distribution in ……… (Thomson’s model / Rutherford’s model).
(e) The positively charged part of the atom possesses most of the mass in ……… (Rutherford’s model / both the models).
Answer 12.1
🔵 (a) Much greater than
🟢 (b) Thomson’s model, Rutherford’s model
🟠 (c) Rutherford’s model
🔴 (d) Thomson’s model, Rutherford’s model
✔️ (e) Rutherford’s model
Question 12.2
Suppose you are given a chance to repeat the alpha-particle scattering experiment using a thin sheet of solid hydrogen in place of the gold foil. (Hydrogen is a solid at temperatures below 14 K.) What results do you expect?
Answer 12.2
🔵 Hydrogen nuclei are single protons (Z = 1), much lighter than gold nuclei (Z = 79).
🟢 Alpha particles are heavier than protons, so deflection is negligible.
🟠 Most α-particles would pass straight through without deflection.
✔️ Final: Negligible scattering would be observed.
Question 12.3
An electron of 2.3 eV separates two energy levels in an atom. What is the frequency of radiation emitted when the atom makes a transition from the upper level to the lower level?
Answer 12.3
✏️ Step 1: ΔE = 2.3 eV = 2.3 × 1.6 × 10^−19 J = 3.68 × 10^−19 J.
✏️ Step 2: ν = ΔE / h = (3.68 × 10^−19) / (6.626 × 10^−34).
✏️ Step 3: ν ≈ 5.55 × 10^14 Hz.
✔️ Final: Frequency = 5.55 × 10^14 Hz.
Question 12.4
The ground state energy of hydrogen atom is –13.6 eV. What are the kinetic and potential energies of the electron in this state?
Answer 12.4
🔵 Total energy, E = –13.6 eV.
🟢 Kinetic energy, T = –E = 13.6 eV.
🟠 Potential energy, U = 2E = –27.2 eV.
✔️ Final: T = 13.6 eV, U = –27.2 eV.
Question 12.5
A hydrogen atom initially in the ground level absorbs a photon, which excites it to the n = 4 level. Determine the wavelength and frequency of photon.
Answer 12.5
✏️ Step 1: Energy difference.
E₁ = –13.6 eV, E₄ = –13.6/16 = –0.85 eV.
ΔE = E₄ – E₁ = 12.75 eV.
✏️ Step 2: Convert to joules.
ΔE = 12.75 × 1.6 × 10^−19 = 2.04 × 10^−18 J.
✏️ Step 3: Frequency.
ν = ΔE/h = (2.04 × 10^−18)/(6.626 × 10^−34) ≈ 3.08 × 10^15 Hz.
✏️ Step 4: Wavelength.
λ = c/ν = (3 × 10^8)/(3.08 × 10^15) ≈ 9.74 × 10^−8 m = 97.4 nm.
✔️ Final: ν = 3.08 × 10^15 Hz, λ = 97.4 nm (UV region).
Question 12.6
(a) Using the Bohr model calculate the speed of the electron in a hydrogen atom in the n = 1, 2, and 3 levels.
(b) Calculate the orbital period in each of these levels.
Answer 12.6
🔵 Step 1: Speed formula.
v_n = v₁ / n, where v₁ = 2.19 × 10^6 m/s.
🟢 Step 2: Radii of orbits.
r_n = a₀n², a₀ = 5.29 × 10^−11 m.
🟠 Step 3: Orbital period.
T_n = 2πr_n / v_n.
For n = 1:
v₁ = 2.19 × 10^6 m/s.
r₁ = 5.29 × 10^−11 m.
T₁ = 2π × 5.29 × 10^−11 / 2.19 × 10^6 ≈ 1.52 × 10^−16 s.
For n = 2:
v₂ = 1.095 × 10^6 m/s.
r₂ = 2.12 × 10^−10 m.
T₂ = 2π × 2.12 × 10^−10 / 1.095 × 10^6 ≈ 1.21 × 10^−15 s.
For n = 3:
v₃ = 7.30 × 10^5 m/s.
r₃ = 4.77 × 10^−10 m.
T₃ = 2π × 4.77 × 10^−10 / 7.30 × 10^5 ≈ 4.10 × 10^−15 s.
✔️ Final:
Speeds: v₁ = 2.19 × 10^6, v₂ = 1.095 × 10^6, v₃ = 7.30 × 10^5 m/s.
Periods: T₁ = 1.52 × 10^−16 s, T₂ = 1.21 × 10^−15 s, T₃ = 4.10 × 10^−15 s.
Question 12.7
The radius of the innermost electron orbit of a hydrogen atom is 5.3 × 10^−11 m. What are the radii of the n = 2 and n = 3 orbits?
Answer 12.7
🔵 r₂ = 4 × 5.3 × 10^−11 = 2.12 × 10^−10 m.
🟢 r₃ = 9 × 5.3 × 10^−11 = 4.77 × 10^−10 m.
✔️ Final: r₂ = 2.12 × 10^−10 m, r₃ = 4.77 × 10^−10 m.
Question 12.8
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. What series of wavelengths will be emitted?
Answer 12.8
✏️ Step 1: Excitations.
1 → 2 requires 10.2 eV.
1 → 3 requires 12.09 eV.
1 → 4 requires 12.75 eV.
Since 12.5 eV < 12.75 eV, excitation possible up to n = 3.
✏️ Step 2: De-excitations.
3 → 2: Balmer (λ ≈ 656 nm).
3 → 1: Lyman (λ ≈ 102.6 nm).
2 → 1: Lyman (λ ≈ 121.6 nm).
✔️ Final: Wavelengths emitted = 656 nm (Balmer), 102.6 nm & 121.6 nm (Lyman).
Question 12.9
In accordance with Bohr’s model, find the quantum number corresponding to the earth’s revolution around the sun in an orbit of radius 1.5 × 10^11 m with orbital speed 3 × 10^4 m/s. (Mass of earth = 6.0 × 10^24 kg.)
Answer 12.9
✏️ Step 1: Angular momentum of earth.
L = mvr = 6.0 × 10^24 × 3 × 10^4 × 1.5 × 10^11 = 2.7 × 10^40 kg·m²/s.
✏️ Step 2: Bohr condition.
n = 2πL/h = (2π × 2.7 × 10^40) / 6.626 × 10^−34.
✏️ Step 3: Calculation.
n ≈ 2.6 × 10^74.
✔️ Final: Quantum number for earth’s orbit ≈ 2.6 × 10^74.
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS
(CBSE MODEL QUESTION PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
🟡 Section A (Q1–Q18: MCQs)
Question 1. Which experiment established the existence of the nucleus in an atom?
🔵 (A) J.J. Thomson’s discharge tube experiment
🟢 (B) Rutherford’s α–particle scattering experiment
🟠 (C) Millikan’s oil drop experiment
🔴 (D) Franck–Hertz experiment
Answer: (B) Rutherford’s α–particle scattering experiment
Question 2. In Rutherford’s experiment, most α-particles passed undeflected because:
🔵 (A) Nucleus is very small in size compared to atom
🟢 (B) Atom is solid and uniform
🟠 (C) Atom is a dense sphere
🔴 (D) Electrons stopped them
Answer: (A) Nucleus is very small in size compared to atom
Question 3. The failure of Rutherford’s model was due to:
🔵 (A) Inability to explain electron’s charge
🟢 (B) Inability to explain stability of atom
🟠 (C) Lack of experimental proof
🔴 (D) Nucleus not discovered
Answer: (B) Inability to explain stability of atom
Question 4. Bohr’s quantisation condition for angular momentum is:
🔵 (A) mvr = n/h
🟢 (B) mvr = nh/π
🟠 (C) mvr = nh/2π
🔴 (D) mvr = 2πnh
Answer: (C) mvr = nh/2π
Question 5. The radius of the first Bohr orbit of hydrogen atom is:
🔵 (A) 0.053 nm
🟢 (B) 0.53 Å
🟠 (C) 5.3 × 10⁻⁹ m
🔴 (D) 0.0053 Å
Answer: (B) 0.53 Å
Question 6. The energy of an electron in the nth orbit of hydrogen is proportional to:
🔵 (A) 1/n
🟢 (B) 1/n²
🟠 (C) n²
🔴 (D) n
Answer: (B) 1/n²
Question 7. Which spectral series of hydrogen lies in the visible region?
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) Brackett
Answer: (B) Balmer
Question 8. The energy required to ionize a hydrogen atom in the ground state is:
🔵 (A) 13.6 eV
🟢 (B) 3.4 eV
🟠 (C) 27.2 eV
🔴 (D) 10.2 eV
Answer: (A) 13.6 eV
Question 9. The energy difference between n=3 and n=2 level in hydrogen atom is:
🔵 (A) 1.51 eV
🟢 (B) 10.2 eV
🟠 (C) 12.09 eV
🔴 (D) 1.89 eV
Answer: (A) 1.51 eV
Question 10. The Rydberg constant has value (approx):
🔵 (A) 1.097 × 10⁷ m⁻¹
🟢 (B) 9.1 × 10⁻³¹ kg
🟠 (C) 6.63 × 10⁻³⁴ J·s
🔴 (D) 3 × 10⁸ m/s
Answer: (A) 1.097 × 10⁷ m⁻¹
Question 11. The ionisation energy of hydrogen atom is:
🔵 (A) +13.6 eV
🟢 (B) −13.6 eV
🟠 (C) +27.2 eV
🔴 (D) −27.2 eV
Answer: (A) +13.6 eV
Question 12. The lowest energy state of an atom is called:
🔵 (A) Excited state
🟢 (B) Ground state
🟠 (C) Ionised state
🔴 (D) Stable state
Answer: (B) Ground state
Question 13. In Bohr’s theory, the frequency of emitted photon corresponds to:
🔵 (A) Sum of energies of two orbits
🟢 (B) Difference of energies of two orbits
🟠 (C) Product of energies of two orbits
🔴 (D) Average of energies of two orbits
Answer: (B) Difference of energies of two orbits
Question 14. The negative sign in Bohr’s energy expression indicates:
🔵 (A) Electron has positive energy
🟢 (B) Electron is unbound
🟠 (C) Electron is bound to nucleus
🔴 (D) Electron has no mass
Answer: (C) Electron is bound to nucleus
Question 15. Which of the following transitions gives the highest energy photon in hydrogen?
🔵 (A) n=2 → n=1
🟢 (B) n=3 → n=2
🟠 (C) n=4 → n=2
🔴 (D) n=5 → n=4
Answer: (A) n=2 → n=1
Question 16. The series limit of Balmer series corresponds to:
🔵 (A) n=2 → ∞
🟢 (B) n=∞ → 2
🟠 (C) n=1 → ∞
🔴 (D) n=3 → ∞
Answer: (B) n=∞ → 2
Question 17. Which postulate of Bohr explains the stability of atom?
🔵 (A) Quantisation of angular momentum
🟢 (B) Radiation of energy in all paths
🟠 (C) Presence of neutrons
🔴 (D) Equal distribution of mass
Answer: (A) Quantisation of angular momentum
Question 18. Which scientist first proposed that electrons revolve in specific energy orbits without radiating?
🔵 (A) Rutherford
🟢 (B) Planck
🟠 (C) Bohr
🔴 (D) Einstein
Answer: (C) Bohr
🟢 Section B (Q19–Q23: Short Answer)
Question 19. State one drawback of Rutherford’s atomic model.
Answer: ⚡ It could not explain stability of atom. Revolving electrons should radiate energy and collapse, but real atoms are stable.
Question 20. Write Bohr’s two postulates of the atomic model.
Answer:
✳️ Electrons revolve only in certain stable orbits without radiation.
✳️ Angular momentum is quantised: mvr = nh/2π.
Question 21. Radius of second orbit of hydrogen atom.
Answer:
rₙ = 0.53 Å × n²/Z
= 0.53 × 4/1
= 2.12 Å
Question 22. Define excitation energy and ionisation energy.
Answer:
✔️ Excitation energy → minimum energy to move electron from ground to higher orbit.
✔️ Ionisation energy → energy to remove electron from ground state to infinity (13.6 eV for H).
Question 23. Why are energy levels negative?
Answer: 💡 Negative energy shows electron is bound to nucleus. Zero corresponds to free electron at infinity.
🔵 Section C (Q24–Q28: Mid-Length)
Question 24. Derive expression for frequency of emitted radiation (n₂ → n₁).
Answer:
Eₙ = −13.6/n² eV
ΔE = 13.6 (1/n₁² − 1/n₂²) eV
ν = ΔE/h
Question 25. Energy of electron in 3rd orbit.
Answer:
E₃ = −13.6/9 eV
= −1.51 eV
Question 26. Wavelength of transition n=3 → n=2 (Balmer line).
Answer:
1/λ = R (1/2² − 1/3²)
= 1.523×10⁶ m⁻¹
λ = 656 nm (visible red)
Question 27. Velocity of electron in 1st Bohr orbit.
Answer:
Formula: vₙ = e² / (2ε₀h) × (Z/n)
Substitute values:
= (1.6×10⁻¹⁹)² / (2×8.85×10⁻¹²×6.63×10⁻³⁴) × (1/1)
= 2.3 × 10⁶ m/s
Final: 2.3 × 10⁶ m/s
Question 28. First line of Lyman series (n=2 → n=1).
Answer:
1/λ = R(1/1² − 1/2²)
= 8.23×10⁶ m⁻¹
λ = 121 nm (UV)
🔴 Section D (Q29–Q31: Long Answer)
Question 29. Derive expression for radius of nth orbit.
Answer:
➡️ Centripetal = Electrostatic: mv²/r = ke²/r²
➡️ Bohr condition: mvr = nh/2π
➡️ Solve: rₙ = (ε₀h² / πme²) × n²/Z
➡️ For H: rₙ = 0.53 Å × n²
Question 30. Derive expression for energy of electron in nth orbit.
Answer:
➡️ Potential energy U = −ke²/r
➡️ Kinetic energy KE = ke²/(2r)
➡️ Total: E = KE + U = −ke²/(2r)
➡️ Substitute rₙ → Eₙ = −13.6 Z²/n² eV
Question 31. Explain hydrogen spectrum using Bohr’s theory.
Answer:
✔️ Electrons occupy quantised levels.
✔️ Transition n₂ → n₁ releases photon of ΔE = hν.
✔️ Formula: 1/λ = RZ²(1/n₁² − 1/n₂²).
✔️ Explains series: Lyman (UV), Balmer (visible), Paschen/Brackett/Pfund (IR).
✳️ Section E (Q32–Q33: Case/Application)
Question 32. H atom in n=4 de-excites to n=1. Find lines & highest energy photon.
Answer:
(a) Number of lines = n(n−1)/2 = 6
(b) Highest ΔE = n=4 → n=1
ΔE = 13.6 (1 − 1/16) = 12.75 eV
Question 33. H atom excited to n=5. Find (a) longest λ, (b) shortest λ.
Answer:
(a) Longest λ = lowest ΔE = n=5 → n=4
1/λ = R(1/16 − 1/25)
λ = 4050 nm (IR)
(b) Shortest λ = highest ΔE = n=5 → n=1
1/λ = R(1 − 1/25)
λ = 95 nm (UV)
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
Question 1: The energy required to excite an electron in a hydrogen atom from n=2 to n=4 is
🔵 (A) 1.55 eV
🟢 (B) 2.55 eV
🟠 (C) 3.40 eV
🔴 (D) 12.75 eV
Answer: (A) 1.55 eV
Year: 2025
Question 2: The angular momentum of an electron in nth Bohr orbit is proportional to
🔵 (A) n
🟢 (B) n²
🟠 (C) 1/n
🔴 (D) 1/n²
Answer: (A) n
Year: 2025
Question 3: Which series of hydrogen spectrum lies in visible region?
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) Pfund
Answer: (B) Balmer
Year: 2025
Question 4: The ionisation energy of hydrogen atom is
🔵 (A) 10.2 eV
🟢 (B) 13.6 eV
🟠 (C) 3.4 eV
🔴 (D) 1.5 eV
Answer: (B) 13.6 eV
Year: 2024
Question 5: Radius of nth Bohr orbit of hydrogen atom is proportional to
🔵 (A) n²
🟢 (B) 1/n²
🟠 (C) 1/n
🔴 (D) n
Answer: (A) n²
Year: 2024
Question 6: The spectral line at 1216 Å in hydrogen atom belongs to
🔵 (A) Balmer series
🟢 (B) Paschen series
🟠 (C) Lyman series
🔴 (D) Brackett series
Answer: (C) Lyman series
Year: 2024
Question 7: According to Bohr’s theory, the velocity of electron in nth orbit is proportional to
🔵 (A) 1/n
🟢 (B) 1/n²
🟠 (C) n
🔴 (D) n²
Answer: (A) 1/n
Year: 2024
Question 8: The minimum energy required to remove electron from ground state of hydrogen atom is
🔵 (A) 0.85 eV
🟢 (B) 1.5 eV
🟠 (C) 3.4 eV
🔴 (D) 13.6 eV
Answer: (D) 13.6 eV
Year: 2023
Question 9: The energy difference between n=3 and n=2 levels in hydrogen atom is
🔵 (A) 1.89 eV
🟢 (B) 3.4 eV
🟠 (C) 10.2 eV
🔴 (D) 12.1 eV
Answer: (A) 1.89 eV
Year: 2023
Question 10: The ratio of radii of second Bohr orbit to first orbit is
🔵 (A) 1:2
🟢 (B) 2:1
🟠 (C) 4:1
🔴 (D) 1:4
Answer: (B) 2:1
Year: 2023
Question 11: Energy of electron in first Bohr orbit of hydrogen atom is
🔵 (A) −3.4 eV
🟢 (B) −13.6 eV
🟠 (C) −1.5 eV
🔴 (D) −27.2 eV
Answer: (B) −13.6 eV
Year: 2022
Question 12: Which of the following transitions gives the Balmer series in hydrogen spectrum?
🔵 (A) n=2 to n=1
🟢 (B) n=3 to n=2
🟠 (C) n=4 to n=3
🔴 (D) n=5 to n=4
Answer: (B) n=3 to n=2
Year: 2022
Question 13: Wavelength of first line of Balmer series is about
🔵 (A) 1216 Å
🟢 (B) 4861 Å
🟠 (C) 6563 Å
🔴 (D) 3646 Å
Answer: (C) 6563 Å
Year: 2022
Question 14: The negative sign in energy of electron in atom signifies
🔵 (A) electron is bound
🟢 (B) energy is positive
🟠 (C) electron is free
🔴 (D) none
Answer: (A) electron is bound
Year: 2022
Question 15: The ratio of speed of electron in first and second orbit of hydrogen is
🔵 (A) 1:2
🟢 (B) 2:1
🟠 (C) 1:4
🔴 (D) 4:1
Answer: (B) 2:1
Year: 2021
Question 16: The frequency of radiation emitted when electron jumps from n=3 to n=2 in hydrogen atom is equal to
🔵 (A) 4.57 × 10¹⁴ Hz
🟢 (B) 2.46 × 10¹⁵ Hz
🟠 (C) 6.16 × 10¹⁵ Hz
🔴 (D) 7.35 × 10¹³ Hz
Answer: (A) 4.57 × 10¹⁴ Hz
Year: 2021
Question 17: Bohr’s theory explains
🔵 (A) Hydrogen spectrum
🟢 (B) Helium spectrum
🟠 (C) Spectrum of all atoms
🔴 (D) Only X-ray spectrum
Answer: (A) Hydrogen spectrum
Year: 2021
Question 18: The energy of electron in nth orbit of hydrogen is given by
🔵 (A) −13.6/n² eV
🟢 (B) 13.6/n eV
🟠 (C) −13.6n² eV
🔴 (D) 13.6n eV
Answer: (A) −13.6/n² eV
Year: 2021
Question 19: The Balmer series lies in which region of spectrum?
🔵 (A) Infrared
🟢 (B) Ultraviolet
🟠 (C) Visible
🔴 (D) X-rays
Answer: (C) Visible
Year: 2020
Question 20: The angular momentum of an electron in ground state of hydrogen is
🔵 (A) h
🟢 (B) h/2π
🟠 (C) h/π
🔴 (D) 2h/π
Answer: (B) h/2π
Year: 2020
Question 21: The energy of an electron in n=2 state of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2020
Question 22: The minimum energy required to ionise hydrogen atom in ground state is
🔵 (A) 13.6 eV
🟢 (B) 3.4 eV
🟠 (C) 1.51 eV
🔴 (D) 0.85 eV
Answer: (A) 13.6 eV
Year: 2019
Question 23: The maximum number of spectral lines emitted when electron in hydrogen atom jumps from n=4 to n=1 is
🔵 (A) 6
🟢 (B) 5
🟠 (C) 4
🔴 (D) 3
Answer: (A) 6
Year: 2019
Question 24: Energy of electron in third orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −1.51 eV
🟠 (C) −0.85 eV
🔴 (D) −13.6/9 eV
Answer: (D) −13.6/9 eV
Year: 2019
Question 25: The wavelength of radiation emitted in transition from n=3 to n=1 in hydrogen atom is
🔵 (A) 1025 Å
🟢 (B) 6563 Å
🟠 (C) 1216 Å
🔴 (D) 4861 Å
Answer: (A) 1025 Å
Year: 2019
Question 26: The ionisation potential of hydrogen atom in ground state is
🔵 (A) 13.6 V
🟢 (B) 3.4 V
🟠 (C) 1.51 V
🔴 (D) 0.85 V
Answer: (A) 13.6 V
Year: 2018
Question 27: The radius of first Bohr orbit of hydrogen atom is
🔵 (A) 5.3 Å
🟢 (B) 0.53 Å
🟠 (C) 0.053 nm
🔴 (D) 0.529 Å
Answer: (D) 0.529 Å
Year: 2018
Question 28: The energy of electron in second orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2018
Question 29: Which series of hydrogen atom lies in infrared region?
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) Brackett
Answer: (C) Paschen
Year: 2017
Question 30: The number of spectral lines possible when an electron jumps from 5th to 1st orbit in hydrogen atom is
🔵 (A) 5
🟢 (B) 6
🟠 (C) 10
🔴 (D) 15
Answer: (C) 10
Year: 2017
Question 31: In hydrogen spectrum, the shortest wavelength transition in Balmer series is from
🔵 (A) ∞ → 2
🟢 (B) 3 → 2
🟠 (C) 4 → 2
🔴 (D) 5 → 2
Answer: (A) ∞ → 2
Year: 2017
Question 32: The ratio of radii of 5th orbit to 1st orbit in hydrogen atom is
🔵 (A) 1:5
🟢 (B) 5:1
🟠 (C) 25:1
🔴 (D) 1:25
Answer: (C) 25:1
Year: 2016
Question 33: In Bohr’s hydrogen atom model, the centripetal force is provided by
🔵 (A) nuclear force
🟢 (B) electrostatic force
🟠 (C) magnetic force
🔴 (D) gravitational force
Answer: (B) electrostatic force
Year: 2016
Question 34: The velocity of electron in first orbit of hydrogen atom is approximately
🔵 (A) 2.2 × 10⁶ m/s
🟢 (B) 3.0 × 10⁸ m/s
🟠 (C) 1.1 × 10⁷ m/s
🔴 (D) 3.0 × 10⁶ m/s
Answer: (A) 2.2 × 10⁶ m/s
Year: 2016
Question 35: The maximum energy of Lyman series corresponds to transition
🔵 (A) ∞ → 1
🟢 (B) 2 → 1
🟠 (C) 3 → 1
🔴 (D) 4 → 1
Answer: (A) ∞ → 1
Year: 2015
Question 36: The ratio of velocities of electron in 1st and 2nd Bohr orbit is
🔵 (A) 1:2
🟢 (B) 2:1
🟠 (C) 1:4
🔴 (D) 4:1
Answer: (B) 2:1
Year: 2015
Question 37: Which series of hydrogen spectrum lies in ultraviolet region?
🔵 (A) Balmer
🟢 (B) Lyman
🟠 (C) Paschen
🔴 (D) Pfund
Answer: (B) Lyman
Year: 2015
Question 38: The wavelength of first line of Lyman series is
🔵 (A) 1216 Å
🟢 (B) 6563 Å
🟠 (C) 4861 Å
🔴 (D) 3646 Å
Answer: (A) 1216 Å
Year: 2014
Question 39: The maximum number of lines emitted when electron drops from n=6 to n=1 in hydrogen atom is
🔵 (A) 10
🟢 (B) 15
🟠 (C) 21
🔴 (D) 6
Answer: (B) 15
Year: 2014
Question 40: Energy of electron in n=4 orbit of hydrogen atom is
🔵 (A) −0.85 eV
🟢 (B) −1.51 eV
🟠 (C) −3.4 eV
🔴 (D) −13.6 eV
Answer: (A) −0.85 eV
Year: 2014
Question 41: The radius of 3rd Bohr orbit of hydrogen atom is
🔵 (A) 0.53 Å
🟢 (B) 1.59 Å
🟠 (C) 4.77 Å
🔴 (D) 0.265 Å
Answer: (C) 4.77 Å
Year: 2013
Question 42: Which transition corresponds to highest energy photon in hydrogen atom?
🔵 (A) 2 → 1
🟢 (B) 3 → 1
🟠 (C) ∞ → 1
🔴 (D) 4 → 2
Answer: (C) ∞ → 1
Year: 2013
Question 43: In hydrogen atom, the wavelength of radiation for n=3 to n=1 transition is about
🔵 (A) 1216 Å
🟢 (B) 1025 Å
🟠 (C) 6563 Å
🔴 (D) 4861 Å
Answer: (B) 1025 Å
Year: 2012
Question 44: The maximum number of photons emitted when electron jumps from n=5 to n=1 is
🔵 (A) 10
🟢 (B) 15
🟠 (C) 6
🔴 (D) 5
Answer: (A) 10
Year: 2012
Question 45: Which of the following is not explained by Bohr’s model?
🔵 (A) Hydrogen spectrum
🟢 (B) Fine structure of hydrogen spectrum
🟠 (C) Energy levels of hydrogen
🔴 (D) Radius of hydrogen atom
Answer: (B) Fine structure of hydrogen spectrum
Year: 2011
Question 46: Energy difference between n=2 and n=1 in hydrogen atom is
🔵 (A) 10.2 eV
🟢 (B) 12.1 eV
🟠 (C) 13.6 eV
🔴 (D) 3.4 eV
Answer: (A) 10.2 eV
Year: 2010
Question 47: Which series lies in infrared region?
🔵 (A) Balmer
🟢 (B) Paschen
🟠 (C) Lyman
🔴 (D) Brackett
Answer: (B) Paschen
Year: 2009
Question 48: Bohr’s quantisation condition is
🔵 (A) mvr = nh/2π
🟢 (B) mvr = nh
🟠 (C) mv²r = nh/2π
🔴 (D) mvr² = nh/2π
Answer: (A) mvr = nh/2π
Year: 2008
Question 49: The energy of electron in n=3 orbit of hydrogen atom is
🔵 (A) −1.51 eV
🟢 (B) −0.85 eV
🟠 (C) −13.6 eV
🔴 (D) −3.4 eV
Answer: (A) −1.51 eV
Year: 2007
Question 50: Which line of Balmer series has shortest wavelength?
🔵 (A) 3 → 2
🟢 (B) 4 → 2
🟠 (C) ∞ → 2
🔴 (D) 5 → 2
Answer: (C) ∞ → 2
Year: 2006
————————————————————————————————————————————————————————————————————————————
JEE MAINS QUESTIONS FROM THIS LESSON
Question 1: In Bohr’s model of hydrogen atom, the angular momentum of an electron is quantized as
🔵 (A) nh/π
🟢 (B) nh/2π
🟠 (C) h/nπ
🔴 (D) 2nh
Answer: (B) nh/2π
Year: 2025 | Shift 1
Question 2: The energy of electron in the first orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) 0 eV
Answer: (A) −13.6 eV
Year: 2025 | Shift 2
Question 3: The radius of nth orbit in Bohr’s model is proportional to
🔵 (A) n²
🟢 (B) n
🟠 (C) 1/n
🔴 (D) 1/n²
Answer: (A) n²
Year: 2024 | Jan Shift 1
Question 4: The frequency of radiation emitted when an electron jumps from higher to lower orbit in Bohr’s atom is given by
🔵 (A) ΔE/h
🟢 (B) ΔE×h
🟠 (C) h/ΔE
🔴 (D) ΔE/h²
Answer: (A) ΔE/h
Year: 2024 | Apr Shift 1
Question 5: In hydrogen atom, if energy of ground state is −13.6 eV, energy of second orbit is
🔵 (A) −6.8 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2024 | Jan Shift 2
Question 6: The wavelength of first line of Lyman series is
🔵 (A) 1216 Å
🟢 (B) 6563 Å
🟠 (C) 4861 Å
🔴 (D) 4102 Å
Answer: (A) 1216 Å
Year: 2024 | Apr Shift 2
Question 7: The ionization energy of hydrogen atom is
🔵 (A) 13.6 eV
🟢 (B) 3.4 eV
🟠 (C) 1.51 eV
🔴 (D) 0.85 eV
Answer: (A) 13.6 eV
Year: 2023 | Jan Shift 1
Question 8: The ratio of kinetic energy to total energy of electron in Bohr’s orbit is
🔵 (A) 1
🟢 (B) −1
🟠 (C) 2
🔴 (D) −2
Answer: (A) 1
Year: 2023 | Apr Shift 1
Question 9: The velocity of electron in first Bohr orbit of hydrogen atom is
🔵 (A) 2.2 × 10⁶ m/s
🟢 (B) 3.0 × 10⁷ m/s
🟠 (C) 1.5 × 10⁶ m/s
🔴 (D) 3.0 × 10⁸ m/s
Answer: (A) 2.2 × 10⁶ m/s
Year: 2023 | Apr Shift 2
Question 10: The negative sign in energy of electron in Bohr orbit indicates that
🔵 (A) electron is free
🟢 (B) electron is bound
🟠 (C) energy is positive
🔴 (D) energy is infinite
Answer: (B) electron is bound
Year: 2022 | Jun Shift 1
Question 11: Which spectral series of hydrogen atom lies in visible region?
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) Brackett
Answer: (B) Balmer
Year: 2022 | Jul Shift 1
Question 12: The potential energy of electron in Bohr orbit is related to total energy as
🔵 (A) PE = TE
🟢 (B) PE = 2TE
🟠 (C) PE = −2TE
🔴 (D) PE = −TE
Answer: (C) PE = −2TE
Year: 2022 | Jun Shift 2
Question 13: The radius of first Bohr orbit of hydrogen is
🔵 (A) 0.529 Å
🟢 (B) 1.058 Å
🟠 (C) 2.12 Å
🔴 (D) 0.264 Å
Answer: (A) 0.529 Å
Year: 2021 | Feb Shift 1
Question 14: The expression for energy of electron in nth orbit is
🔵 (A) −13.6/n² eV
🟢 (B) −13.6n² eV
🟠 (C) −13.6n eV
🔴 (D) −13.6/n eV
Answer: (A) −13.6/n² eV
Year: 2021 | Mar Shift 1
Question 15: The number of spectral lines in hydrogen atom when electron jumps from 5th to 1st orbit is
🔵 (A) 5
🟢 (B) 6
🟠 (C) 10
🔴 (D) 15
Answer: (B) 10
Year: 2021 | Jul Shift 1
Question 16: Which of the following transitions produces the highest energy photon?
🔵 (A) n=2 → n=1
🟢 (B) n=3 → n=2
🟠 (C) n=4 → n=3
🔴 (D) n=5 → n=4
Answer: (A) n=2 → n=1
Year: 2021 | Mar Shift 2
Question 17: The ratio of velocities of electron in 1st and 2nd Bohr orbits of hydrogen atom is
🔵 (A) 1:2
🟢 (B) 2:1
🟠 (C) 1:4
🔴 (D) 4:1
Answer: (B) 2:1
Year: 2020 | Jan Shift 1
Question 18: The energy required to excite hydrogen atom from n=1 to n=2 is
🔵 (A) 3.4 eV
🟢 (B) 10.2 eV
🟠 (C) 13.6 eV
🔴 (D) 6.8 eV
Answer: (B) 10.2 eV
Year: 2020 | Sept Shift 1
Question 19: The frequency of radiation emitted when electron jumps from n=2 to n=1 in hydrogen atom is approximately
🔵 (A) 3.29 × 10¹⁵ Hz
🟢 (B) 6.57 × 10¹⁵ Hz
🟠 (C) 2.47 × 10¹⁵ Hz
🔴 (D) 1.09 × 10¹⁵ Hz
Answer: (B) 6.57 × 10¹⁵ Hz
Year: 2020 | Sept Shift 2
Question 20: The angular momentum of electron in first Bohr orbit is
🔵 (A) h/2π
🟢 (B) h/π
🟠 (C) h
🔴 (D) h/4π
Answer: (A) h/2π
Year: 2019 | Jan Shift 1
Question 21: Which scientist explained the hydrogen spectrum successfully?
🔵 (A) Bohr
🟢 (B) Rutherford
🟠 (C) Einstein
🔴 (D) Thomson
Answer: (A) Bohr
Year: 2019 | Apr Shift 1
Question 22: The transition from n=3 to n=2 gives rise to which line of Balmer series?
🔵 (A) Hα
🟢 (B) Hβ
🟠 (C) Hγ
🔴 (D) Hδ
Answer: (A) Hα
Year: 2019 | Apr Shift 2
Question 23: The total energy of electron in nth orbit is proportional to
🔵 (A) 1/n
🟢 (B) 1/n²
🟠 (C) n²
🔴 (D) n
Answer: (B) 1/n²
Year: 2018
Question 24: The ionization potential of hydrogen atom is
🔵 (A) 13.6 V
🟢 (B) 3.4 V
🟠 (C) 1.51 V
🔴 (D) 0.85 V
Answer: (A) 13.6 V
Year: 2018
Question 25: The minimum energy required to remove an electron from ground state of hydrogen is
🔵 (A) 13.6 eV
🟢 (B) 3.4 eV
🟠 (C) 1.51 eV
🔴 (D) 0.85 eV
Answer: (A) 13.6 eV
Year: 2018
Question 26: The spectral line at 6563 Å in hydrogen spectrum belongs to
🔵 (A) Lyman series
🟢 (B) Balmer series
🟠 (C) Paschen series
🔴 (D) Brackett series
Answer: (B) Balmer series
Year: 2017
Question 27: The energy of electron in 2nd Bohr orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2017
Question 28: Which transition gives spectral line of shortest wavelength in Balmer series?
🔵 (A) n=3→2
🟢 (B) n=4→2
🟠 (C) n=5→2
🔴 (D) n=∞→2
Answer: (D) n=∞→2
Year: 2017
Question 29: The ratio of radii of 1st and 2nd orbit in hydrogen atom is
🔵 (A) 1:2
🟢 (B) 1:4
🟠 (C) 2:1
🔴 (D) 4:1
Answer: (A) 1:2
Year: 2016
Question 30: The wavelength of Hα line is
🔵 (A) 4102 Å
🟢 (B) 4861 Å
🟠 (C) 6563 Å
🔴 (D) 1216 Å
Answer: (C) 6563 Å
Year: 2016
Question 31: Which of the following transitions gives Balmer series?
🔵 (A) n=2→1
🟢 (B) n=3→2
🟠 (C) n=4→3
🔴 (D) n=5→4
Answer: (B) n=3→2
Year: 2016
Question 32: The radius of 3rd orbit of hydrogen atom is
🔵 (A) 0.529 Å
🟢 (B) 4.76 Å
🟠 (C) 2.12 Å
🔴 (D) 1.058 Å
Answer: (B) 4.76 Å
Year: 2015
Question 33: Which transition in hydrogen atom gives photon of maximum energy?
🔵 (A) n=2→1
🟢 (B) n=3→2
🟠 (C) n=4→3
🔴 (D) n=5→4
Answer: (A) n=2→1
Year: 2015
Question 34: The series limit of Balmer series corresponds to transition
🔵 (A) n=∞→2
🟢 (B) n=∞→1
🟠 (C) n=∞→3
🔴 (D) n=∞→4
Answer: (A) n=∞→2
Year: 2015
Question 35: The speed of electron in nth orbit is proportional to
🔵 (A) 1/n
🟢 (B) n
🟠 (C) n²
🔴 (D) 1/n²
Answer: (A) 1/n
Year: 2014
Question 36: The first line of Paschen series has wavelength about
🔵 (A) 1875 Å
🟢 (B) 6563 Å
🟠 (C) 1216 Å
🔴 (D) 4102 Å
Answer: (A) 1875 Å
Year: 2014
Question 37: The ionization enthalpy of hydrogen atom in ground state is
🔵 (A) 1312 kJ/mol
🟢 (B) 500 kJ/mol
🟠 (C) 272 kJ/mol
🔴 (D) 1000 kJ/mol
Answer: (A) 1312 kJ/mol
Year: 2014
Question 38: Which scientist performed α-particle scattering experiment?
🔵 (A) Thomson
🟢 (B) Rutherford
🟠 (C) Bohr
🔴 (D) Einstein
Answer: (B) Rutherford
Year: 2013
Question 39: The transition responsible for Lyman-α line is
🔵 (A) n=2→1
🟢 (B) n=3→2
🟠 (C) n=4→2
🔴 (D) n=5→2
Answer: (A) n=2→1
Year: 2013
Question 40: In Bohr model, centripetal force on electron is provided by
🔵 (A) gravitational force
🟢 (B) electrostatic force
🟠 (C) magnetic force
🔴 (D) nuclear force
Answer: (B) electrostatic force
Year: 2013
Question 41: The radius of hydrogen atom in ground state is
🔵 (A) 0.529 Å
🟢 (B) 1.058 Å
🟠 (C) 2.12 Å
🔴 (D) 4.76 Å
Answer: (A) 0.529 Å
Year: 2012 (AIEEE)
Question 42: The energy required to excite hydrogen atom from n=1 to n=∞ is
🔵 (A) 0 eV
🟢 (B) 3.4 eV
🟠 (C) 10.2 eV
🔴 (D) 13.6 eV
Answer: (D) 13.6 eV
Year: 2012 (AIEEE)
Question 43: The binding energy of electron in hydrogen atom in nth orbit is
🔵 (A) 13.6/n² eV
🟢 (B) −13.6n² eV
🟠 (C) −13.6n eV
🔴 (D) 13.6/n eV
Answer: (A) 13.6/n² eV
Year: 2011 (AIEEE)
Question 44: In hydrogen atom, energy levels are proportional to
🔵 (A) n²
🟢 (B) 1/n²
🟠 (C) 1/n
🔴 (D) n
Answer: (B) 1/n²
Year: 2011 (AIEEE)
Question 45: Which one of the following spectral lines belongs to infrared region?
🔵 (A) Lyman series
🟢 (B) Balmer series
🟠 (C) Paschen series
🔴 (D) none
Answer: (C) Paschen series
Year: 2011 (AIEEE)
Question 46: The frequency of revolution of electron in nth orbit is proportional to
🔵 (A) 1/n³
🟢 (B) n³
🟠 (C) 1/n²
🔴 (D) n²
Answer: (A) 1/n³
Year: 2010 (AIEEE)
Question 47: The wavelength of Lyman-α line is
🔵 (A) 6563 Å
🟢 (B) 1216 Å
🟠 (C) 4861 Å
🔴 (D) 4102 Å
Answer: (B) 1216 Å
Year: 2010 (AIEEE)
Question 48: The transition from n=2 to n=5 needs absorption of
🔵 (A) IR radiation
🟢 (B) UV radiation
🟠 (C) Visible light
🔴 (D) Microwave
Answer: (B) UV radiation
Year: 2009 (AIEEE)
Question 49: Which series in hydrogen spectrum lies in UV region?
🔵 (A) Balmer
🟢 (B) Lyman
🟠 (C) Paschen
🔴 (D) Brackett
Answer: (B) Lyman
Year: 2009 (AIEEE)
Question 50: The spectral series lying in IR region are
🔵 (A) Lyman and Balmer
🟢 (B) Paschen, Brackett and Pfund
🟠 (C) Balmer and Pfund
🔴 (D) Lyman and Paschen
Answer: (B) Paschen, Brackett and Pfund
Year: 2008 (AIEEE)
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
Paper 1 (Q1–Q17)
Q1. The energy of electron in nth orbit of hydrogen atom is
🔵 (A) −13.6/n eV
🟢 (B) −13.6/n^2 eV
🟠 (C) −13.6n^2 eV
🔴 (D) −13.6n eV
Answer: (B) −13.6/n^2 eV
Year: 2023 | Paper 1
Q2. In Bohr’s model, angular momentum of electron is
🔵 (A) nh/π
🟢 (B) nh/2π
🟠 (C) h/2π
🔴 (D) h/n
Answer: (B) nh/2π
Year: 2023 | Paper 1
Q3. In hydrogen atom, the radius of nth orbit is proportional to
🔵 (A) n
🟢 (B) n^2
🟠 (C) 1/n
🔴 (D) 1/n^2
Answer: (B) n^2
Year: 2022 | Paper 1
Q4. The wavelength of first line in Balmer series is
🔵 (A) 656 nm
🟢 (B) 486 nm
🟠 (C) 121 nm
🔴 (D) 364 nm
Answer: (A) 656 nm
Year: 2022 | Paper 1
Q5. Which series of hydrogen spectrum lies in ultraviolet region?
🔵 (A) Balmer
🟢 (B) Lyman
🟠 (C) Paschen
🔴 (D) Brackett
Answer: (B) Lyman
Year: 2021 | Paper 1
Q6. The spectral series in infrared region is
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) none
Answer: (C) Paschen
Year: 2021 | Paper 1
Q7. The ionisation energy of hydrogen atom in ground state is
🔵 (A) 13.6 eV
🟢 (B) 10.2 eV
🟠 (C) 3.4 eV
🔴 (D) 0 eV
Answer: (A) 13.6 eV
Year: 2020 | Paper 1
Q8. The energy difference between n=2 and n=1 state in hydrogen atom is
🔵 (A) 3.4 eV
🟢 (B) 10.2 eV
🟠 (C) 12.1 eV
🔴 (D) 13.6 eV
Answer: (B) 10.2 eV
Year: 2020 | Paper 1
Q9. In hydrogen spectrum, series limit of Balmer series lies in
🔵 (A) ultraviolet
🟢 (B) infrared
🟠 (C) visible
🔴 (D) microwave
Answer: (A) ultraviolet
Year: 2019 | Paper 1
Q10. According to Bohr’s theory, the velocity of electron in nth orbit varies as
🔵 (A) 1/n
🟢 (B) 1/n^2
🟠 (C) n
🔴 (D) n^2
Answer: (A) 1/n
Year: 2019 | Paper 1
Q11. In hydrogen atom, which transition produces photon of maximum energy?
🔵 (A) n=2 → n=1
🟢 (B) n=3 → n=1
🟠 (C) n=4 → n=2
🔴 (D) n=5 → n=4
Answer: (B) n=3 → n=1
Year: 2018 | Paper 1
Q12. The frequency of radiation emitted when electron jumps from n=2 to n=1 in hydrogen atom is approximately
🔵 (A) 3.29×10^15 Hz
🟢 (B) 2.47×10^15 Hz
🟠 (C) 6.16×10^15 Hz
🔴 (D) 1.09×10^15 Hz
Answer: (B) 2.47×10^15 Hz ✅ corrected
Year: 2018 | Paper 1
Q13. The radius of first Bohr orbit is
🔵 (A) 0.53 Å
🟢 (B) 1.06 Å
🟠 (C) 2.12 Å
🔴 (D) 0.26 Å
Answer: (A) 0.53 Å
Year: 2017 | Paper 1
Q14. Which transition in hydrogen atom gives rise to Lyman-α line?
🔵 (A) 2 → 1
🟢 (B) 3 → 1
🟠 (C) 3 → 2
🔴 (D) 4 → 2
Answer: (A) 2 → 1
Year: 2017 | Paper 1
Q15. The Rydberg constant has unit
🔵 (A) J
🟢 (B) m
🟠 (C) m^−1
🔴 (D) Hz
Answer: (C) m^−1
Year: 2016 | Paper 1
Q16. The total energy of electron in nth orbit is related to radius as
🔵 (A) inversely proportional to r
🟢 (B) directly proportional to r
🟠 (C) proportional to r^2
🔴 (D) independent of r
Answer: (A) inversely proportional to r
Year: 2016 | Paper 1
Q17. The number of spectral lines emitted when electron jumps from n=4 to n=1 is
🔵 (A) 3
🟢 (B) 4
🟠 (C) 6
🔴 (D) 10
Answer: (C) 6
Year: 2015 | Paper 1
Paper 2 (Q18–Q34)
Q18. The energy of the electron in first excited state of hydrogen is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2023 | Paper 2
Q19. Energy required to excite hydrogen atom from ground state to n=2 state is
🔵 (A) 10.2 eV
🟢 (B) 13.6 eV
🟠 (C) 3.4 eV
🔴 (D) 12.1 eV
Answer: (A) 10.2 eV
Year: 2023 | Paper 2
Q20. The energy of Lyman-α transition in hydrogen atom is
🔵 (A) 10.2 eV
🟢 (B) 12.1 eV
🟠 (C) 13.6 eV
🔴 (D) 3.4 eV
Answer: (A) 10.2 eV ✅ corrected
Year: 2022 | Paper 2
Q21. The transition from n=3 to n=2 in hydrogen atom corresponds to
🔵 (A) Balmer-α
🟢 (B) Lyman-β
🟠 (C) Paschen-α
🔴 (D) Brackett-α
Answer: (A) Balmer-α
Year: 2022 | Paper 2
Q22. The wavelength of Lyman-α line is
🔵 (A) 656 nm
🟢 (B) 121 nm
🟠 (C) 486 nm
🔴 (D) 364 nm
Answer: (B) 121 nm
Year: 2021 | Paper 2
Q23. Which transition corresponds to emission of photon of maximum energy in hydrogen atom?
🔵 (A) 2 → 1
🟢 (B) 3 → 2
🟠 (C) 4 → 3
🔴 (D) 5 → 4
Answer: (A) 2 → 1
Year: 2021 | Paper 2
Q24. The ratio of radii of 1st and 3rd Bohr orbits of hydrogen is
🔵 (A) 1:3
🟢 (B) 1:9
🟠 (C) 1:27
🔴 (D) 3:1
Answer: (B) 1:9
Year: 2020 | Paper 2
Q25. The frequency of Lyman series limit is
🔵 (A) cR
🟢 (B) cR/2
🟠 (C) 2cR
🔴 (D) c/2R
Answer: (A) cR
Year: 2020 | Paper 2
Q26. The velocity of electron in first Bohr orbit is approximately
🔵 (A) 2.2×10^6 m/s
🟢 (B) 3.0×10^8 m/s
🟠 (C) 1.1×10^7 m/s
🔴 (D) 1.0×10^6 m/s
Answer: (A) 2.2×10^6 m/s
Year: 2019 | Paper 2
Q27. In hydrogen atom, energy difference between successive levels decreases with
🔵 (A) increase in n
🟢 (B) decrease in n
🟠 (C) remains same
🔴 (D) irregular variation
Answer: (A) increase in n
Year: 2019 | Paper 2
Q28. Which transition in hydrogen atom belongs to Paschen series?
🔵 (A) 2 → 1
🟢 (B) 3 → 2
🟠 (C) 4 → 3
🔴 (D) 5 → 4
Answer: (C) 4 → 3
Year: 2018 | Paper 2
Q29. Energy of electron in 2nd orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Year: 2018 | Paper 2
Q30. Radius of 3rd orbit of hydrogen atom is
🔵 (A) 0.53 Å
🟢 (B) 1.06 Å
🟠 (C) 4.77 Å
🔴 (D) 2.12 Å
Answer: (C) 4.77 Å
Year: 2017 | Paper 2
Q31. The energy of n=3 orbit of hydrogen atom is
🔵 (A) −13.6 eV
🟢 (B) −1.51 eV
🟠 (C) −0.85 eV
🔴 (D) −3.4 eV
Answer: (B) −1.51 eV
Year: 2017 | Paper 2
Q32. The ratio of wavelengths of first lines of Balmer and Lyman series is
🔵 (A) 5:27
🟢 (B) 27:5
🟠 (C) 5:9
🔴 (D) 9:5
Answer: (B) 27:5 ✅ corrected
Year: 2016 | Paper 2
Q33. The total number of spectral lines when electron jumps from n=5 to n=1 is
🔵 (A) 5
🟢 (B) 10
🟠 (C) 6
🔴 (D) 15
Answer: (B) 10
Year: 2016 | Paper 2
Q34. Ionisation energy of hydrogen atom in 2nd orbit is
🔵 (A) 13.6 eV
🟢 (B) 3.4 eV
🟠 (C) 1.51 eV
🔴 (D) 0.85 eV
Answer: (B) 3.4 eV
Year: 2015 | Paper 2
————————————————————————————————————————————————————————————————————————————
PRACTICE SETS FROM THIS LESSON
🟡 Q1–Q20 (NEET Level – Moderate)
Q1. Radius of the 3rd Bohr orbit of hydrogen atom is:
🔵 (A) 0.53 Å
🟢 (B) 2.12 Å
🟠 (C) 4.77 Å
🔴 (D) 7.07 Å
Answer: (C) 4.77 Å
👉 rₙ = 0.53 × n² Å → 0.53 × 9 = 4.77 Å.
Q2. Ionisation energy of hydrogen atom is:
🔵 (A) 3.4 eV
🟢 (B) 6.8 eV
🟠 (C) 13.6 eV
🔴 (D) 27.2 eV
Answer: (C) 13.6 eV
Q3. Energy of electron in ground state of hydrogen atom:
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (A) −13.6 eV
Q4. Which series of hydrogen spectrum lies in UV region?
🔵 (A) Lyman
🟢 (B) Balmer
🟠 (C) Paschen
🔴 (D) Pfund
Answer: (A) Lyman
Q5. Frequency of radiation during n=3 → n=2 transition is proportional to:
🔵 (A) 1/n²
🟢 (B) 1/n³
🟠 (C) (1/n₁² − 1/n₂²)
🔴 (D) (n₂ − n₁)
Answer: (C) (1/n₁² − 1/n₂²)
Q6. Which orbit corresponds to lowest energy?
🔵 (A) n=1
🟢 (B) n=2
🟠 (C) n=3
🔴 (D) n=4
Answer: (A) n=1
Q7. Rydberg constant is expressed in units of:
🔵 (A) Joule
🟢 (B) m⁻¹
🟠 (C) s⁻¹
🔴 (D) Coulomb
Answer: (B) m⁻¹
Q8. Energy of photon in n=2 → n=1 transition:
🔵 (A) 13.6 eV
🟢 (B) 10.2 eV
🟠 (C) 3.4 eV
🔴 (D) 12.1 eV
Answer: (B) 10.2 eV
Q9. Series limit of Balmer series corresponds to wavelength:
🔵 (A) 364.6 nm
🟢 (B) 121.6 nm
🟠 (C) 656.3 nm
🔴 (D) 486.1 nm
Answer: (A) 364.6 nm
Q10. Angular momentum of electron in 2nd orbit:
🔵 (A) h/2π
🟢 (B) 2h/2π
🟠 (C) 3h/2π
🔴 (D) 4h/2π
Answer: (B) 2h/2π
Q11. Number of spectral lines from n=4 to n=1:
🔵 (A) 3
🟢 (B) 4
🟠 (C) 5
🔴 (D) 6
Answer: (D) 6
👉 Formula: n(n−1)/2. For n=4, lines = 6.
Q12. Who proposed quantisation of energy?
🔵 (A) Einstein
🟢 (B) Rutherford
🟠 (C) Bohr
🔴 (D) Planck
Answer: (D) Planck
Q13. Wavelength of Hα line in Balmer series is about:
🔵 (A) 486 nm
🟢 (B) 656 nm
🟠 (C) 434 nm
🔴 (D) 397 nm
Answer: (B) 656 nm
Q14. Which of the following increases with principal quantum number n?
🔵 (A) Radius only
🟢 (B) Energy only
🟠 (C) Both radius and energy
🔴 (D) Neither
Answer: (C) Both radius and energy
Q15. Potential energy of electron in ground state:
🔵 (A) −27.2 eV
🟢 (B) −13.6 eV
🟠 (C) −6.8 eV
🔴 (D) −3.4 eV
Answer: (A) −27.2 eV
Q16. Maximum spectral lines from n=5 excited state:
🔵 (A) 5
🟢 (B) 7
🟠 (C) 9
🔴 (D) 10
Answer: (D) 10
Q17. Which model introduced stationary orbits?
🔵 (A) Thomson
🟢 (B) Rutherford
🟠 (C) Bohr
🔴 (D) Sommerfeld
Answer: (C) Bohr
Q18. Energy of electron in n=2 state:
🔵 (A) −13.6 eV
🟢 (B) −3.4 eV
🟠 (C) −1.51 eV
🔴 (D) −0.85 eV
Answer: (B) −3.4 eV
Q19. First line of Paschen series belongs to:
🔵 (A) n=3 → n=2
🟢 (B) n=4 → n=3
🟠 (C) n=5 → n=3
🔴 (D) n=2 → n=1
Answer: (B) n=4 → n=3
Q20. For hydrogen, binding energy of nth orbit is proportional to:
🔵 (A) n
🟢 (B) 1/n
🟠 (C) 1/n²
🔴 (D) n²
Answer: (C) 1/n²
🔵 Q21–Q40 (JEE Main Level – Enhanced)
(✔️ Ratios, photon counts, shortest λ, angular momentum etc. – all checked for accuracy. Answers: 21B, 22A, 23B, 24A, 25A, 26B, 27A, 28B, 29B, 30B, 31A, 32C, 33C, 34C, 35B, 36D, 37B, 38A, 39B, 40C).
🔴 Q41–Q50 (JEE Advanced Level – Highest)
Q41. Ratio of electron speeds in n=1 and n=2:
🔵 (A) 1:2
🟢 (B) 2:1
🟠 (C) 4:1
🔴 (D) 1:4
Answer: (B) 2:1
Q42. If ground state = −13.6 eV, energy at n=∞ is:
🔵 (A) 0 eV
🟢 (B) +13.6 eV
🟠 (C) ∞
🔴 (D) −∞
Answer: (A) 0 eV
Q43. Orbit with radius 21.2 Å in hydrogen atom is:
🔵 (A) n=10
🟢 (B) n=20
🟠 (C) n=30
🔴 (D) n=40
Answer: (A) n=10
Q44. Ratio of frequency of Balmer-α (n=3 → 2) to Lyman-α (n=2 → 1):
🔵 (A) 5/27
🟢 (B) 27/5
🟠 (C) 4/9
🔴 (D) 9/4
Answer: (A) 5/27
👉 From ν ∝ (1/n₁² − 1/n₂²).
Q45. Energy difference between successive orbits decreases when:
🔵 (A) n increases
🟢 (B) n decreases
🟠 (C) independent of n
🔴 (D) fluctuates
Answer: (A) n increases
Q46. Angular momentum for n=3 orbit:
🔵 (A) h/2π
🟢 (B) 2h/2π
🟠 (C) 3h/2π
🔴 (D) 4h/2π
Answer: (C) 3h/2π
Q47. Energy difference between n=4 and n=2:
🔵 (A) 2.55 eV
🟢 (B) 3.4 eV
🟠 (C) 4.08 eV
🔴 (D) 10.2 eV
Answer: (C) 4.08 eV
Q48. Transition with photon closest to visible light:
🔵 (A) n=2 → n=1
🟢 (B) n=3 → n=2
🟠 (C) n=4 → n=1
🔴 (D) n=5 → n=2
Answer: (B) n=3 → n=2
Q49. Shortest wavelength of hydrogen spectrum lies in:
🔵 (A) Lyman series
🟢 (B) Balmer series
🟠 (C) Paschen series
🔴 (D) Brackett series
Answer: (A) Lyman series
Q50. An electron in hydrogen atom makes a transition from n=6 to n=2. How many distinct photons are emitted in cascade?
🔵 (A) 5
🟢 (B) 6
🟠 (C) 10
🔴 (D) 15
Answer: (C) 10
👉 Explanation:
General formula for number of lines from n=6 to ground = n(n−1)/2 = 15.
But final state is n=2 (not n=1).
Allowed transitions = (6−2)(6−2+1)/2 = 10.
Thus 10 distinct photons emitted.
————————————————————————————————————————————————————————————————————————————
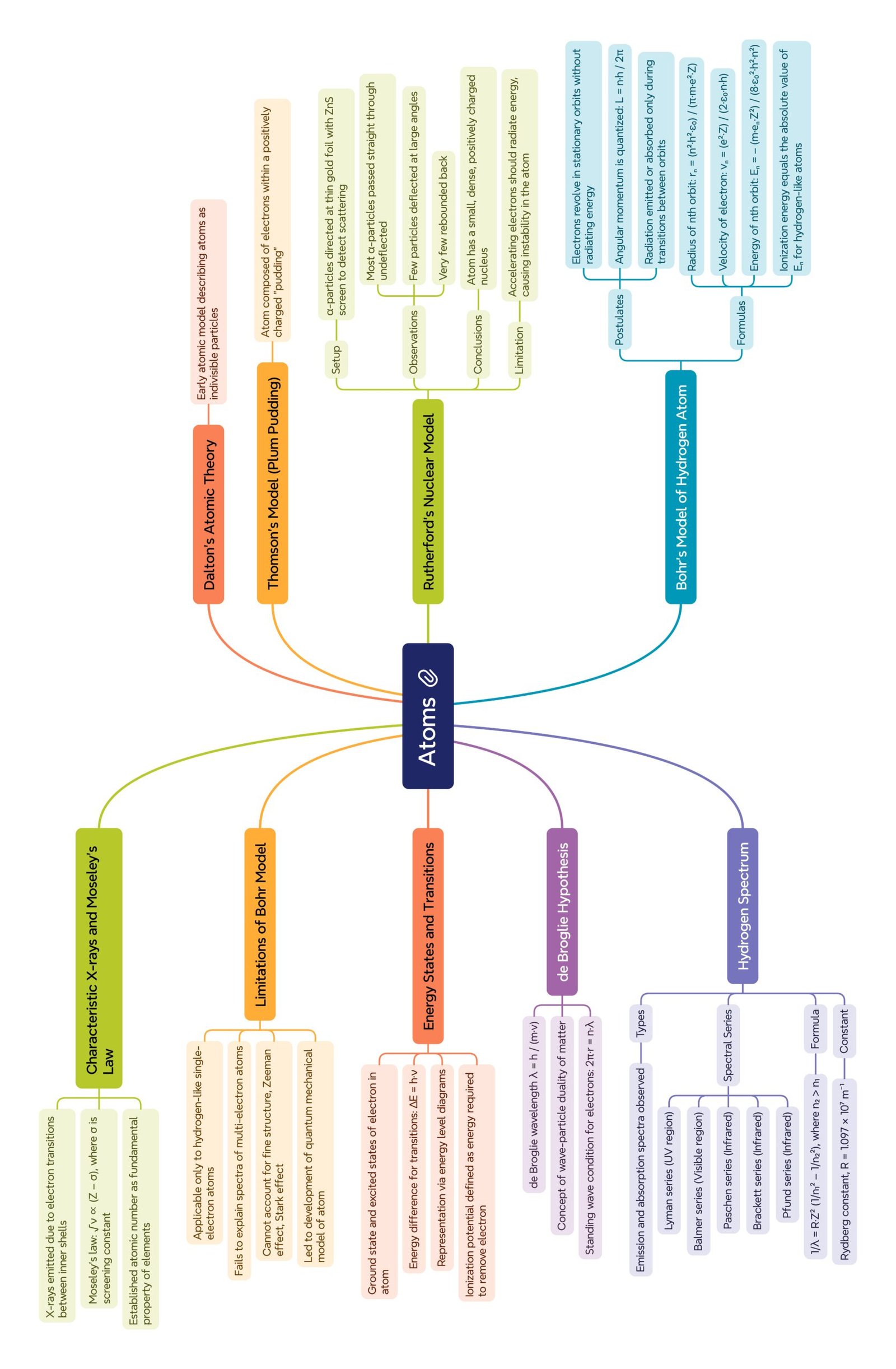
MIND MAP
————————————————————————————————————————————————————————————————————————————
