Class 12 : Chemistry (English) – Chapter 1: Solutions
EXPLANATION & SUMMARY
This chapter primarily deals with different types of solutions, the methods to express their concentration, the concept of solubility, the colligative properties of solutions, and Raoult’s law. Here’s a complete explanation of all concepts covered in this lesson in clear and systematic order:
TYPES OF SOLUTIONS
A solution is a homogeneous mixture of two or more substances. The component present in the largest amount is usually called the solvent, and the other is the solute.
Types of Solutions Based on Physical States:
Gaseous Solutions: Gas in gas (air), gas in liquid (oxygen in water)
Liquid Solutions: Liquid in liquid (ethanol in water), solid in liquid (salt in water)
Solid Solutions: Solid in solid (alloys like brass – zinc in copper)
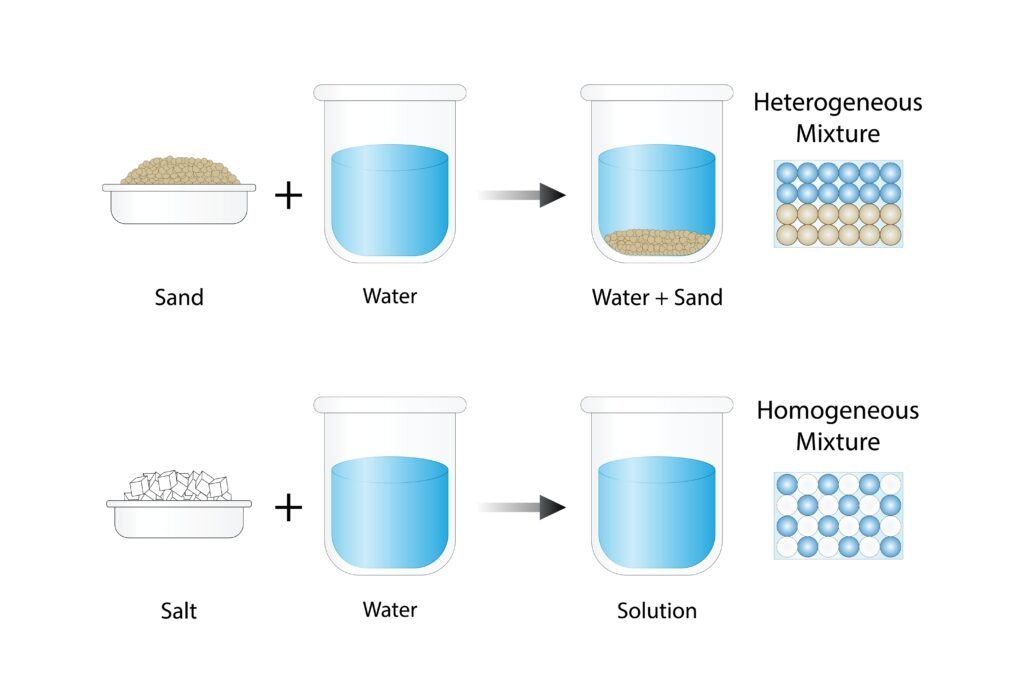
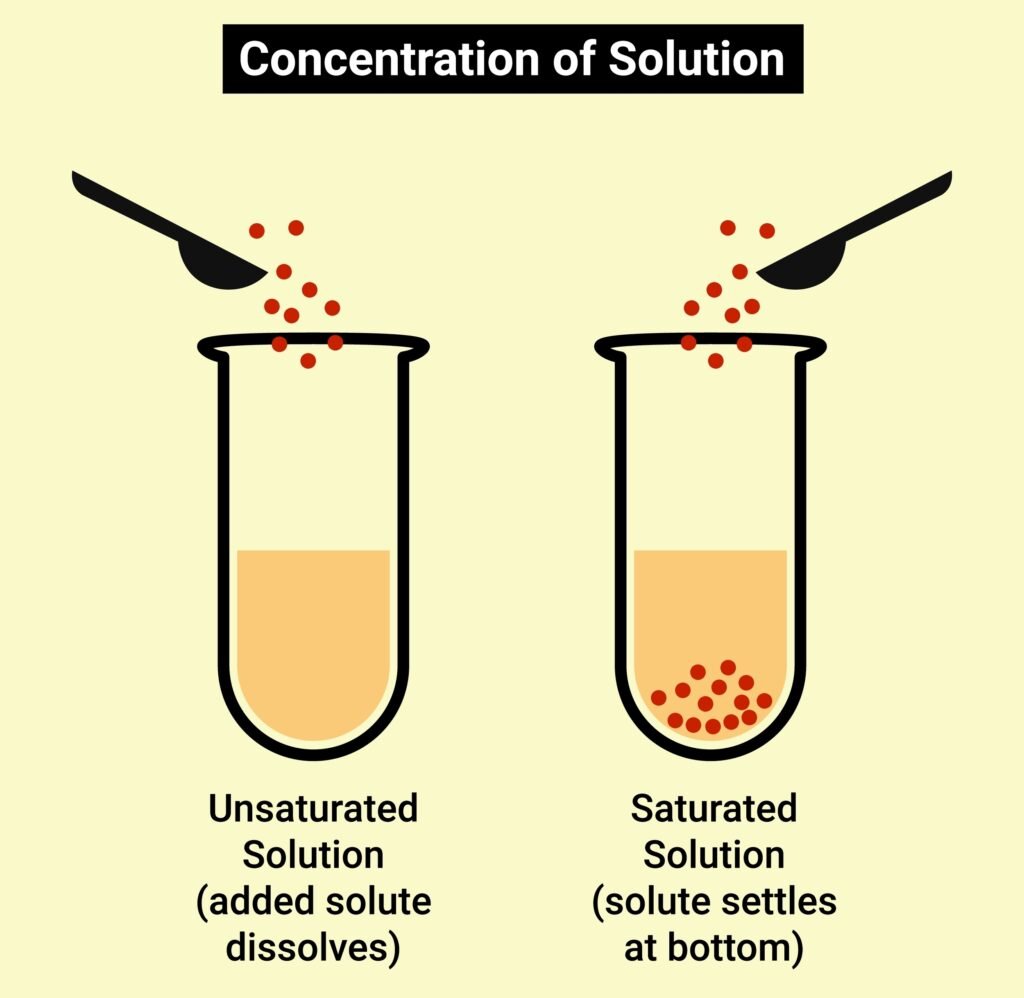
EXPRESSING CONCENTRATION OF SOLUTIONS
The concentration of a solution tells how much solute is present in a given quantity of solvent or solution.
Common ways to express concentration:
a. Mass Percentage (w/w):
Mass of solute / Mass of solution × 100
b. Volume Percentage (v/v):
Volume of solute / Volume of solution × 100
c. Mass by Volume Percentage (w/v):
Mass of solute / Volume of solution × 100
d. Parts per million (ppm):
Used for very dilute solutions.
Mass of solute / Mass of solution × 10⁶
e. Mole Fraction (χ):
Moles of component / Total moles of all components
If there are only two components A and B:
χA + χB = 1
f. Molarity (M):
Moles of solute / Volume of solution in litres
g. Molality (m):
Moles of solute / Mass of solvent in kg
Molality is temperature-independent.
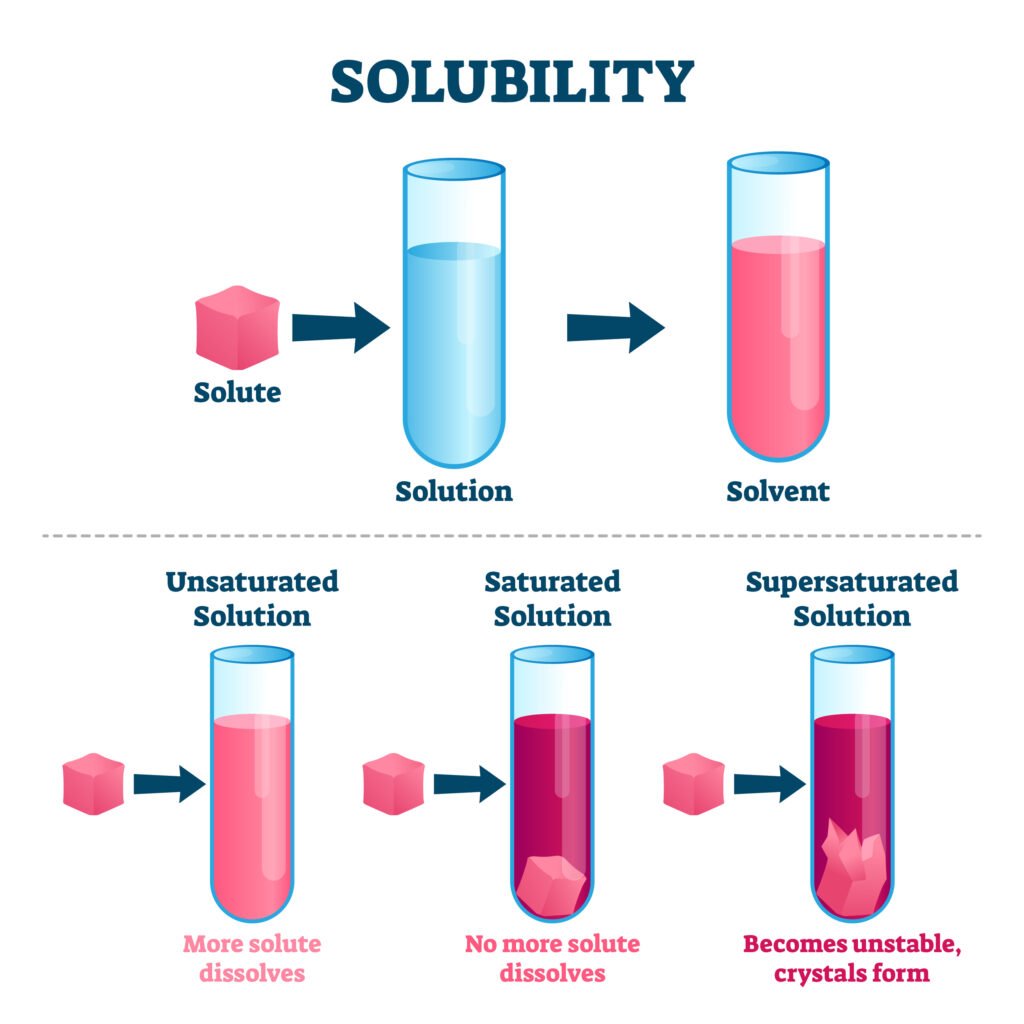
SOLUBILITY
Solubility refers to the maximum amount of a solute that can dissolve in a specific amount of solvent at a specific temperature.
Factors Affecting Solubility:
a. Nature of solute and solvent:
“Like dissolves like” – polar solutes dissolve in polar solvents.
b. Temperature:
For solids in liquids: solubility generally increases with temperature.
For gases in liquids: solubility decreases with temperature.
c. Pressure (Henry’s Law):
The solubility of a gas in a liquid is directly proportional to the pressure of the gas.
Henry’s Law: p = KH × x
Where p = partial pressure of gas, x = mole fraction in solution, KH = Henry’s constant.
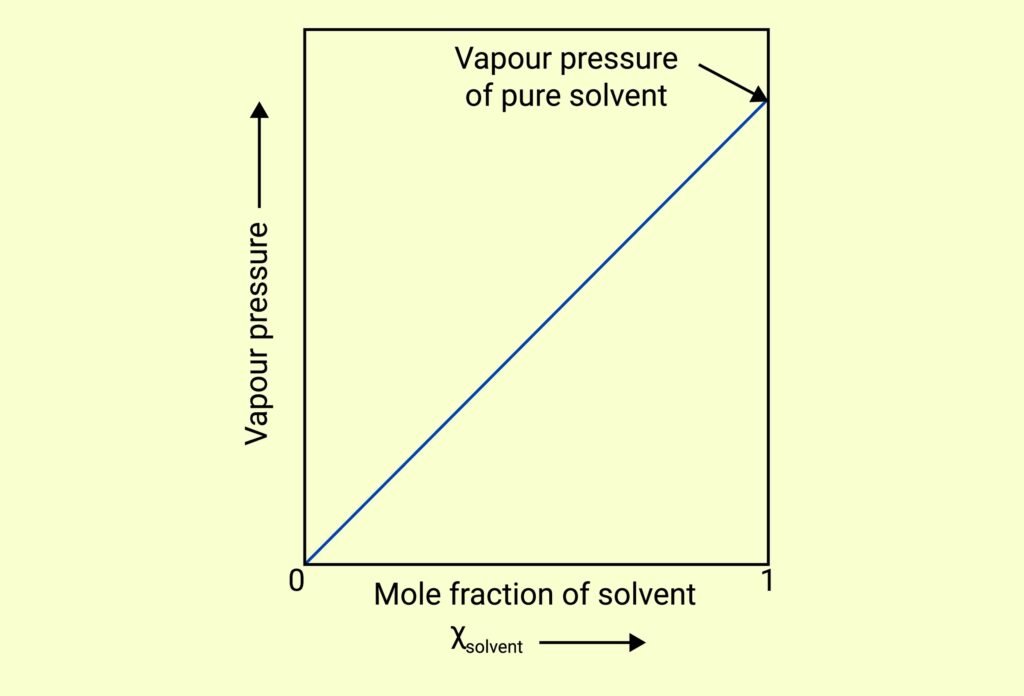
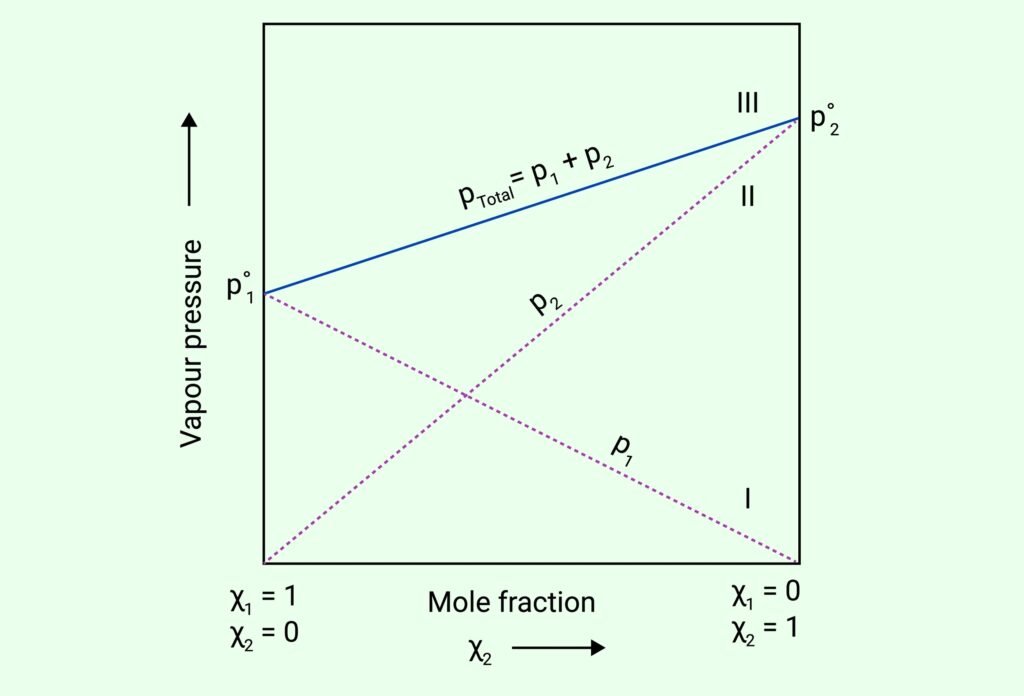
VAPOUR PRESSURE OF LIQUID SOLUTIONS
Raoult’s Law (for volatile solutes):
For a solution of two volatile liquids A and B:
pA = pA° × xA, pB = pB° × xB
Total vapour pressure:
pTotal = pA + pB = pA°xA + pB°xB
Where:
pA° and pB° are vapour pressures of pure components,
xA and xB are mole fractions.
If solute is non-volatile, only the solvent contributes to vapour pressure:
pSolution = xSolvent × p°Solvent
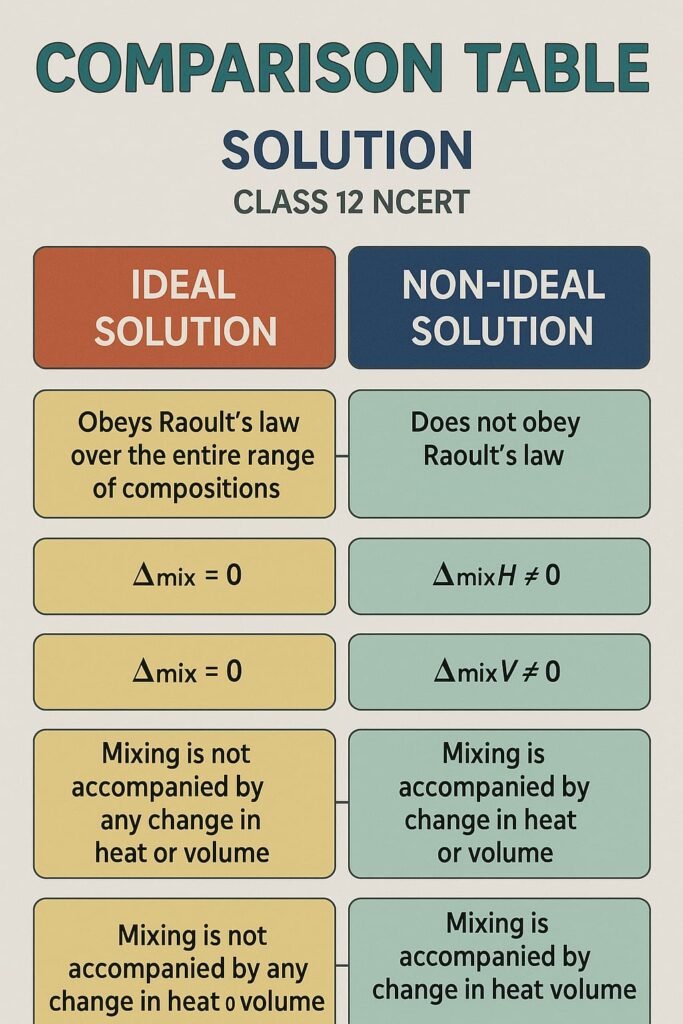
Ideal Solutions: Follow Raoult’s Law at all compositions; no enthalpy change or volume change.
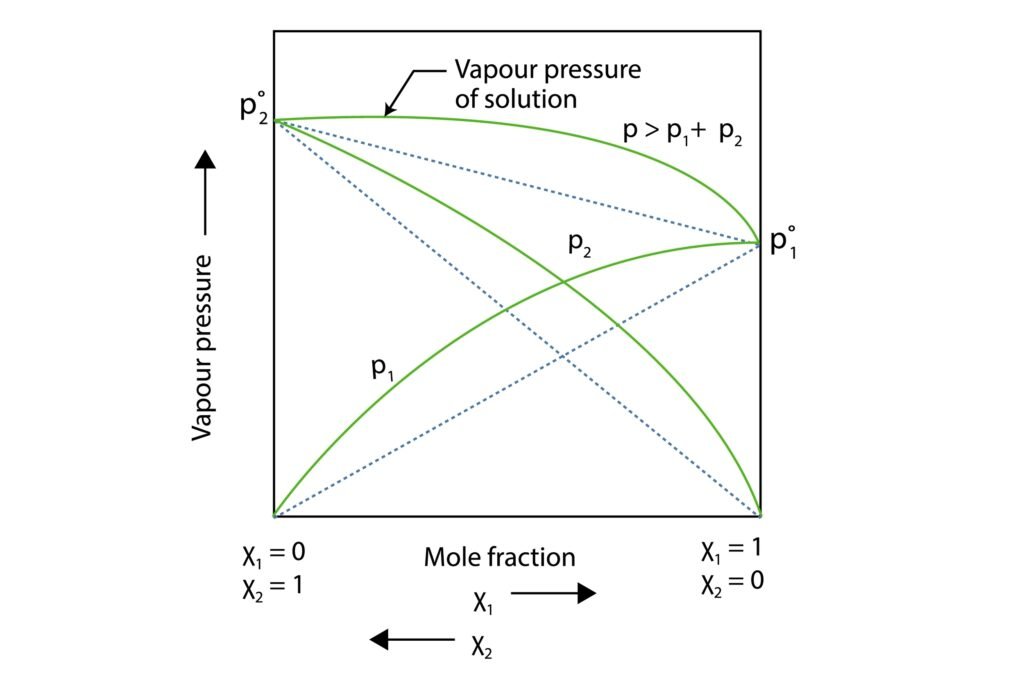
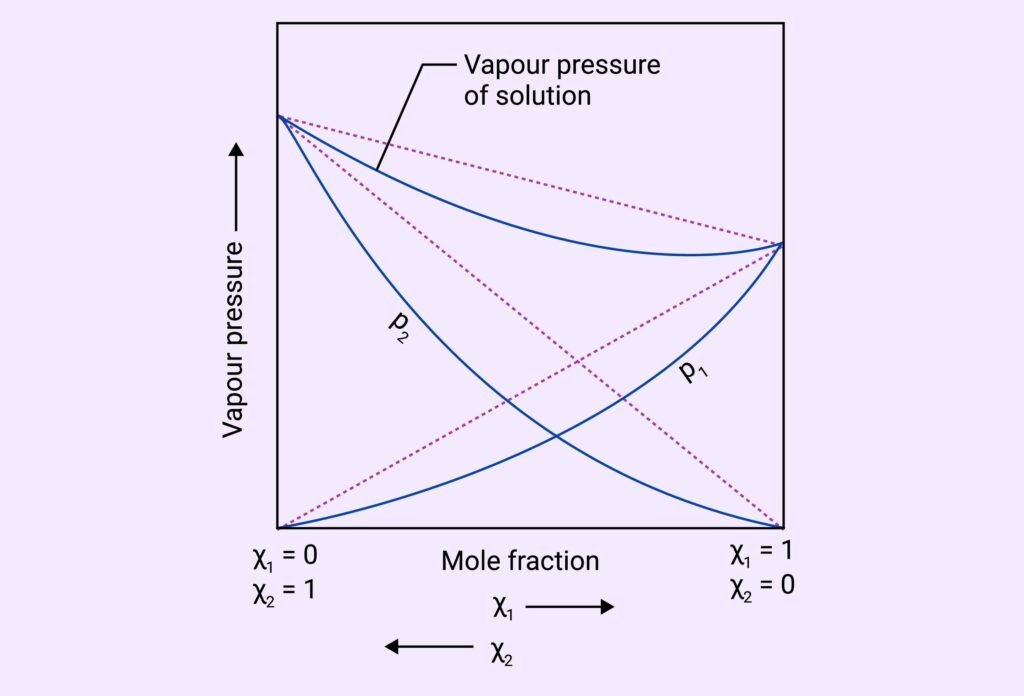
Non-Ideal Solutions: Deviate from Raoult’s Law.
Positive Deviation: vapour pressure more than predicted (weak A-B interactions)
Negative Deviation: vapour pressure less than predicted (strong A-B interactions)
COLLIGATIVE PROPERTIES
Colligative properties depend only on the number of solute particles and not their nature.
There are four types:
(a) Relative Lowering of Vapour Pressure (RLVP)
For a solution with non-volatile solute:
(p⁰ – p) / p⁰ = xSolute = n₂ / (n₁ + n₂)
If n₂ ≪ n₁, then
(p⁰ – p) / p⁰ ≈ n₂ / n₁
(b) Elevation of Boiling Point (ΔTb)
Boiling point of solution > pure solvent
ΔTb = Kb × m
Where:
Kb = ebullioscopic constant
m = molality
(c) Depression of Freezing Point (ΔTf)
Freezing point of solution < pure solvent
ΔTf = Kf × m
Where:
Kf = cryoscopic constant
m = molality
(d) Osmotic Pressure (π)
π = CRT
Where:
π = osmotic pressure
C = molar concentration
R = gas constant (0.0821 L·atm/K·mol)
T = absolute temperature
Osmosis: flow of solvent through a semipermeable membrane from dilute to concentrated solution.
Reverse Osmosis: Used in water purification (RO systems)
VAN’T HOFF FACTOR (i)
Used when the solute undergoes association or dissociation.
i = Actual number of particles in solution / Expected number of particles
i affects all colligative properties:
ΔTb, ΔTf, π, RLVP ∝ i
Examples:
NaCl → 2 ions (i ≈ 2)
BaCl₂ → 3 ions (i ≈ 3)
Glucose → no dissociation (i = 1)
Corrected formulae:
ΔTb = i × Kb × m
ΔTf = i × Kf × m
π = i × C × R × T
(p⁰ – p) / p⁰ = i × xSolute
ABNORMAL MOLECULAR MASSES
Colligative property-based molecular mass may differ due to:
Dissociation: apparent molar mass < actual
Association: apparent molar mass > actual
Corrected Molecular Mass:
Mobserved = Mtheoretical / i
PRACTICAL APPLICATIONS
Antifreeze in car radiators: Ethylene glycol lowers freezing point.
Salting roads: Salt lowers freezing point of water.
IV Fluids: Must be isotonic to blood plasma to avoid osmotic effects.
Reverse Osmosis (RO): Uses osmotic pressure to purify water.
Food preservation: Salt/sugar creates high osmotic pressure preventing microbial growth.
CONCEPTUAL CLARIFICATIONS
Difference between Molality and Molarity:
Molarity changes with temperature (volume changes), molality does not.
Ideal vs Non-Ideal Solutions:
Ideal: No volume or heat change on mixing
Non-Ideal: Show enthalpy or volume change
Why colligative properties are useful:
They help determine molecular mass of solute, detect abnormal behaviour like dissociation, and are used in biological and industrial applications.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
Question 1.1:
Define the term solution. How many types of solutions are formed? Write briefly about each type with an example.
Answer:
A solution is a homogeneous mixture of two or more components uniformly dispersed throughout a single phase.
Types of solutions based on physical states:
Solid in solid – Example: Brass (copper + zinc alloy).
Gas in liquid – Example: Carbonated water (CO₂ dissolved in H₂O).
Liquid in liquid – Example: Ethanol in water.
Gas in gas – Example: Air (mixture of N₂ and O₂).
Question 1.2:
Give an example of a solid solution in which the solute is a gas.
Answer:
Hydrogen gas dissolved in palladium metal (Pd–H system).
Question 1.3:
Define the following terms:
(i) Mole fraction
(ii) Molality
(iii) Molarity
(iv) Mass percentage
Answer:
(i) Mole fraction (xᵢ) = nᵢ / Σnⱼ
(ii) Molality (m) = moles of solute / mass of solvent in kg
(iii) Molarity (M) = moles of solute / volume of solution in litres
(iv) Mass % = (mass of solute / mass of solution) × 100
Question 1.4:
Concentrated nitric acid is 68% HNO₃ by mass, density 1.504 g/mL. What is its molarity?
Answer:
In 100 g solution:
HNO₃ = 68 g; Molar mass = 63 g/mol ⇒ Moles = 68/63 = 1.079 mol
Volume = 100/1.504 = 66.49 mL = 0.06649 L
Molarity = 1.079 / 0.06649 ≈ 16.23 M
Question 1.5:
A 10% w/w glucose solution has density 1.2 g/mL. Find its molality, mole fraction, and molarity.
Answer:
In 100 g solution: Glucose = 10 g ⇒ Moles = 10/180 = 0.0556 mol
Water = 90 g = 0.090 kg ⇒ Molality = 0.0556 / 0.090 = 0.618 m
Moles of water = 90/18 = 5 mol
Mole fraction of glucose = 0.0556 / (0.0556 + 5) = 0.0111
Molarity = 0.0556 / (100/1.2 ÷ 1000) = 0.0556 / 0.08333 = 0.667 M
Question 1.6:
How many mL of 0.1 M HCl are needed to react with 1 g mixture of equimolar Na₂CO₃ and NaHCO₃?
Answer:
Average molar mass = (106 + 84)/2 = 95 g/mol ⇒ Moles = 1/95 ≈ 0.0105 mol
Each salt = 0.00525 mol
Na₂CO₃ requires 2 mol HCl/mol ⇒ 0.00525×2 = 0.0105 mol
NaHCO₃ requires 1 mol HCl/mol ⇒ 0.00525×1 = 0.00525 mol
Total HCl = 0.01575 mol ⇒ Volume = 0.01575 / 0.1 = 157.5 mL
Question 1.7:
300 g of 25% solution is mixed with 400 g of 40% solution. Find mass % of final solution.
Answer:
Solute: 300×0.25 = 75 g, 400×0.40 = 160 g ⇒ Total = 235 g
Total mass = 700 g ⇒ Mass % = (235/700)×100 = 33.57%
Question 1.8:
Antifreeze: 222.6 g C₂H₆O₂ + 200 g H₂O; density = 1.072 g/mL. Find molality and molarity.
Answer:
Molar mass of C₂H₆O₂ = 62 g/mol ⇒ Moles = 222.6/62 = 3.593 mol
Molality = 3.593 / 0.200 = 17.97 m
Total volume = 422.6 / 1.072 = 394.2 mL = 0.3942 L
Molarity = 3.593 / 0.3942 ≈ 9.12 M
Question 1.9:
Water contains 15 ppm CHCl₃. Find its mass % and molality.
Answer:
15 ppm = 15 mg/kg = 1.5×10⁻³%
Molality = (15×10⁻³ g) / 119.38 g/mol = 1.256×10⁻⁴ mol/kg = 1.256×10⁻⁴ m
Question 1.10:
Discuss the role of molecular interactions in alcohol–water solution.
Answer:
Alcohol forms hydrogen bonds with water. These interactions are different from pure components and cause deviations from ideality, affecting properties like vapor pressure and boiling point.
Question 1.11:
Why do gases become less soluble in liquids at higher temperatures?
Answer:
Solubility of gases decreases with temperature because added heat allows gas molecules to escape from the solvent (based on Le Châtelier’s principle).
Question 1.12:
State Henry’s law and mention its applications.
Answer:
Henry’s law: p = k·x (pressure is proportional to mole fraction of gas in liquid).
Applications:
Carbonated drinks
Scuba diving (avoiding bends)
Industrial gas absorption
Question 1.13:
Mass of ethane = 6.56×10⁻³ g exerts 1 bar pressure. What is the pressure for 5×10⁻² g?
Answer:
Pressure ∝ mass ⇒
p = 1 × (5×10⁻² / 6.56×10⁻³) = 7.62 bar
Question 1.14:
What are positive and negative deviations from Raoult’s law? How are they related to ΔHₘᵢₓ?
Answer:
Positive deviation: weaker A–B interactions, higher vapor pressure, ΔHₘᵢₓ > 0
Negative deviation: stronger A–B interactions, lower vapor pressure, ΔHₘᵢₓ < 0
Question 1.15:
A 2% non-volatile solute solution exerts 1.004 bar vapor pressure at boiling point. Find molar mass.
Answer:
ΔT_b = 0.004 K; K_b = 0.52 ⇒ m = ΔT_b/K_b = 0.00769 mol/kg
Molality = (2/M)/0.098 ⇒ 0.00769 = (2/M)/0.098 ⇒ M ≈ 260–264 g/mol
Question 1.16:
Ideal solution: 26 g heptane (M=100) and 35 g octane (M=114); p⁰₁ = 105.2 kPa, p⁰₂ = 46.8 kPa. Find vapor pressure.
Answer:
n₁ = 0.26 mol, n₂ = 0.307 mol
x₁ = 0.458, x₂ = 0.542
p = 0.458×105.2 + 0.542×46.8 = 76.7 kPa
Question 1.17:
Vapor pressure of water = 12.3 kPa at 300 K. What is vapor pressure of 1 molal solute solution?
Answer:
x_solute = 1 / (1 + 55.5) = 0.0177
Δp = 12.3 × 0.0177 = 0.218 kPa
New vapor pressure = 12.3 – 0.218 = 12.08 kPa
Question 1.18:
Find mass of solute (M = 40 g/mol) in 114 g octane to reduce vapor pressure to 80%.
Answer:
p/p₀ = x_solvent = 0.80 ⇒ n_solute = (1/0.80 – 1) = 0.25 mol
mass = 0.25 × 40 = 10 g
Question 1.19:
Solution: 30 g solute in 90 g water gives p = 2.8 kPa; after adding 18 g water, p = 2.9 kPa.
(i) Find molar mass of solute
(ii) Find vapor pressure of pure water
Answer:
Let M = molar mass, p₀ = vapor pressure of water
First case: n_sol = 30/M, n_H₂O = 90/18 = 5 mol
p₁ = p₀ × (5 / (5 + 30/M)) = 2.8
Second case: n_H₂O = (90+18)/18 = 6 mol
p₂ = p₀ × (6 / (6 + 30/M)) = 2.9
Divide equations and solve: M ≈ 60 g/mol, p₀ ≈ 3.2 kPa
Question 1.20:
Freezing point of 5% sugar (M = 342 g/mol) is 271 K. Find freezing point of 5% glucose (M = 180 g/mol).
Answer:
m_glucose = (5/180)/0.095 = 0.293 m
ΔT_f = 1.86 × 0.293 = 0.545 K
Freezing point = 273.15 – 0.545 = 272.61 K
Question 1.21:
Two elements A and B form compounds AB₂ and AB₄. When 1 g of AB₂ and AB₄ are each dissolved in 20 g benzene, AB₂ lowers the freezing point by 2.3 K and AB₄ by 1.3 K. Kf for benzene = 5.1 K kg mol⁻¹. Calculate atomic masses of A and B.
Answer:
For AB₂:
M₁ = (5.1 × 1000 × 1) / (2.3 × 20) = 110.87 g/mol
For AB₄:
M₂ = (5.1 × 1000 × 1) / (1.3 × 20) = 196.15 g/mol
Let A = x, B = y
Then,
x + 2y = 110.87
x + 4y = 196.15
Subtracting: 2y = 85.28 → y = 42.64 g/mol
x = 110.87 − 85.28 = 25.59 g/mol
Question 1.22:
A 0.5% (w/w) aqueous KCl solution freezes at −0.24°C. Find the van’t Hoff factor (i) and degree of dissociation (α). (Kf = 1.86 K kg mol⁻¹)
Answer:
Mass of solvent = 99.5 g = 0.0995 kg
Molality = (0.5 / 74.5) / 0.0995 = 0.0675 mol/kg
i = ΔTf / (Kf × molality) = 0.24 / (1.86 × 0.0675) = 1.91
Since KCl dissociates into K⁺ and Cl⁻:
i = 1 + α ⇒ α = i − 1 = 0.91 or 91%
Question 1.23:
Solubility of H₂S in water at STP is 0.195 mol/kg. Calculate Henry’s law constant.
Answer:
Mole fraction of H₂S ≈ 0.195 / (0.195 + 55.5) ≈ 0.00351
KH = P / x = 1 / 0.00351 = 285 bar
Question 1.24:
Henry’s law constant for CO₂ in water at 298 K is 1.67 × 10⁸ Pa. How much CO₂ is dissolved in 500 mL soda water under 2.5 atm CO₂ pressure?
Answer:
P = 2.5 atm = 2.5 × 101325 = 253312.5 Pa
x = 253312.5 / 1.67 × 10⁸ = 1.517 × 10⁻³
Moles of water = 500 / 18 = 27.78 mol
Moles of CO₂ = 1.517 × 10⁻³ × 27.78 = 0.0421 mol
Mass = 0.0421 × 44 = 1.85 g
Question 1.25:
P°A = 450 mmHg, P°B = 700 mmHg, total pressure = 600 mmHg. Find liquid and vapour phase compositions.
Answer:
600 = xA × 450 + (1 − xA) × 700
Solving: xA = 0.4, xB = 0.6
yA = (xA × P°A) / Ptotal = (0.4 × 450) / 600 = 0.3
yB = 1 − yA = 0.7
Question 1.26:
Vapour pressure of water at 298 K is 23.8 mmHg. 50 g urea in 850 g water. Find vapour pressure of solution and relative lowering.
Answer:
Moles urea = 50 / 60 = 0.833
Moles water = 850 / 18 = 47.22
xH₂O = 47.22 / (47.22 + 0.833) = 0.9827
Vapour pressure = 0.9827 × 23.8 = 23.39 mmHg
Relative lowering = (23.8 − 23.39) / 23.8 = 0.0173
Question 1.27:
Water boils at 99.63°C at 750 mmHg. Find mass of sucrose to be added to 500 g water to raise b.p. to 100°C. (Kb = 0.52 K kg mol⁻¹)
Answer:
ΔTb = 0.37 K
Molality = 0.37 / 0.52 = 0.712 mol/kg
Moles = 0.712 × 0.5 = 0.356
Mass = 0.356 × 342 = 121.75 g
Question 1.28:
Find mass of ascorbic acid (M = 176 g/mol) to dissolve in 75 g acetic acid to lower melting point by 1.5°C. (Kf = 3.9)
Answer:
Molality = 1.5 / 3.9 = 0.385
Moles = 0.385 × 0.075 = 0.0289
Mass = 0.0289 × 176 = 5.08 g
Question 1.29:
4 g MgSO₄ (M = 120) in 100 g water. Calculate boiling point. Assume complete ionisation. (Kb = 0.52)
Answer:
Molality = (4 / 120) / 0.1 = 0.333
i = 2 ⇒ ΔTb = 2 × 0.52 × 0.333 = 0.346
Boiling point = 100 + 0.346 = 100.35°C
Question 1.30:
1.75 g Na₂SO₄ in 50 g water. Calculate freezing point. (Kf = 1.86)
Answer:
Molality = (1.75 / 142) / 0.05 = 0.246
i = 3 ⇒ ΔTf = 3 × 1.86 × 0.246 = 1.374
Freezing point = 0 − 1.374 = −1.374°C
Question 1.31:
How much CaCl₂ (i = 2.47) in 2.5 L water to get π = 0.75 atm at 27°C?
Answer:
T = 300 K, R = 0.0821
0.75 = 2.47 × M × 0.0821 × 300
M = 0.0123 mol/L
Moles = 0.0123 × 2.5 = 0.0308
Mass = 0.0308 × 111 = 3.42 g
Question 1.32:
25 mg K₂SO₄ in 2 L water at 25°C. Find osmotic pressure.
Answer:
Moles = 0.025 / 174 = 1.437 × 10⁻⁴
Molarity = 7.18 × 10⁻⁵
i = 3
π = 3 × 7.18 × 10⁻⁵ × 0.0821 × 298 = 5.27 × 10⁻³ atm
Question 1.33:
Find molarity of sugar solution with same osmotic pressure as 0.1 M NaCl at 300 K.
Answer:
i(NaCl) = 2 ⇒ effective concentration = 0.2 M
Sugar (i = 1): molarity = 0.2 M
Question 1.34:
1 g polymer (M = 185000) in 450 mL water at 37°C. Find osmotic pressure in pascals.
Answer:
Moles = 1 / 185000 = 5.41 × 10⁻⁶
Molarity = 5.41 × 10⁻⁶ / 0.45 = 1.20 × 10⁻⁵
π = M × R × T = 1.20 × 10⁻⁵ × 8.314 × 310 = 0.031 Pa
Question 1.35:
30 g solute in 90 g water: p = 2.8 kPa. After 18 g water added: p = 2.9 kPa. Find (i) molar mass (ii) vapour pressure of water.
Answer:
Initial: n_sol = 30/M, n_water = 90/18 = 5 mol
Final: water = 108 g → 6 mol
Using Raoult’s law and ratio of vapour pressures:
M ≈ 60 g/mol, P₀ ≈ 3.4 kPa
Question 1.36:
5% cane sugar lowers freezing point to 271 K. What is freezing point of 5% glucose solution?
Answer:
ΔTf = 2.15 K, m (sugar) = 0.154
Kf = 2.15 / 0.154 ≈ 14
Glucose: m = (5 / 180) / 0.095 = 0.292
ΔTf = 14 × 0.292 = 4.09
Freezing point = 273.15 − 4.09 = 269.06 K
Question 1.37:
Mix 480 mL of 1.5 M and 520 mL of 1.2 M solution. Find final molarity.
Answer:
Moles = (1.5 × 0.48) + (1.2 × 0.52) = 1.344
Volume = 1.0 L ⇒ Molarity = 1.344 M
Question 1.38:
A 2% solute solution exerts 1.004 bar at boiling point. Find molar mass.
Answer:
Relative lowering = (1.013 − 1.004)/1.013 = 0.00888
n_water = 98 / 18 = 5.44
(2 / M) / 5.44 = 0.00888 ⇒ M = 41.4 g/mol
Question 1.39:
Heptane: 26 g (M = 100), Octane: 35 g (M = 114), P₀ = 105.2 and 46.8 kPa. Find vapour pressure.
Answer:
n_heptane = 0.26, n_octane = 0.307
x_heptane = 0.458, x_octane = 0.542
P_total = (0.458 × 105.2) + (0.542 × 46.8) = 76.7 kPa
Question 1.40:
7 g protein in 100 mL has π = 25 mmHg at 37°C. Find molar mass.
Answer:
π = 25 / 760 = 0.0329 atm
T = 310 K
Molarity = π / (R × T) = 1.29 × 10⁻³ mol/L
Molar mass = 7 / (1.29 × 10⁻³ × 0.1) = 54264 g/mol
Question 1.41:
25 mg K₂SO₄ in 2 L at 25°C. Find osmotic pressure.
Answer:
Moles = 0.025 / 174 = 1.437 × 10⁻⁴
Molarity = 7.18 × 10⁻⁵
π = 3 × 7.18 × 10⁻⁵ × 0.0821 × 298 = 5.27 × 10⁻³ atm
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
(CBSE MODEL QUESTIONS PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
SECTION A (1 MARK EACH)
Answer the following questions briefly.
Q.1 Define molality.
Answer: Molality is the number of moles of solute dissolved per kilogram of solvent.
Q.2 What is the van’t Hoff factor for NaCl in aqueous solution?
Answer: 2
Q.3 What type of deviation from Raoult’s law is shown by a solution formed by mixing acetone and chloroform?
Answer: Negative deviation
Q.4 State Henry’s law.
Answer: Henry’s law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas.
Q.5 Which will have a higher boiling point: 1 molal NaCl or 1 molal glucose solution? Why?
Answer: 1 molal NaCl, because it ionizes to give more particles, causing higher elevation in boiling point.
Q.6 What happens to the solubility of gases in liquids with increase in temperature?
Answer: It decreases.
Q.7 What is an ideal solution?
Answer: A solution that obeys Raoult’s law at all concentrations and temperatures.
Q.8 Which colligative property is best suited to determine the molar mass of a solute?
Answer: Depression in freezing point
Q.9 Give an example of a solution showing positive deviation from Raoult’s law.
Answer: Ethanol and acetone
Q.10 Write the relationship between mole fraction of a component and partial pressure in a binary solution.
Answer: pA = xA × pA⁰
SECTION B (2 MARKS EACH)
Answer the following in about 30–40 words.
Q.11 Explain why elevation of boiling point is considered a colligative property.
Answer: It depends only on the number of solute particles, not their nature. More particles lower vapor pressure and raise boiling point.
QWhy do aquatic species face difficulty in breathing in warm water?
Answer: The solubility of oxygen gas decreases with rise in temperature, so less oxygen is available in warm water.
Q.12 Define osmotic pressure. Write the formula.
Answer: Osmotic pressure is the minimum pressure required to stop osmosis.
π = C × R × T, where C = molarity, R = gas constant, T = temperature.
Q.13 How does adding a non-volatile solute to a solvent affect vapor pressure?
Answer: It lowers the vapor pressure due to reduced number of solvent molecules at the surface.
Q.14 What is meant by abnormal molar mass? How is it caused?
Answer: When observed molar mass deviates from theoretical value due to association or dissociation of solute in solution.
SECTION C (3 MARKS EACH)
Answer the following in about 50–60 words.
Q.15 A solution contains 5 g of urea (molar mass = 60 g/mol) in 100 g of water. Calculate the lowering of vapor pressure if the vapor pressure of pure water is 32 mmHg.
Answer:
Moles of urea = 5/60 = 0.083 mol
Moles of water = 100/18 = 5.56 mol
Mole fraction of solute = 0.083 / (0.083 + 5.56) ≈ 0.0147
Lowering of vapor pressure = 32 × 0.0147 ≈ 0.47 mmHg
Q.16 What is Raoult’s law for a binary liquid solution?
Answer:
Raoult’s law states that the partial vapor pressure of each component is directly proportional to its mole fraction:
pA = xA × pA⁰,
pB = xB × pB⁰,
Total vapor pressure = pA + pB
Q.17 State two differences between ideal and non-ideal solutions with examples.
Answer:
Ideal: Obey Raoult’s law (e.g., benzene + toluene)
Non-ideal: Deviate from Raoult’s law (e.g., acetone + chloroform)
Ideal solutions show no enthalpy change; non-ideal ones do.
Q.18 Explain the effect of pressure and temperature on solubility of gases in liquids.
Answer:
Pressure: Solubility increases with pressure (Henry’s law).
Temperature: Solubility decreases with temperature due to increased kinetic energy.
Q.19 Define colligative properties. Name four such properties.
Answer:
Colligative properties depend on the number of solute particles, not their type.
Examples:
(i) Relative lowering of vapor pressure
(ii) Elevation of boiling point
(iii) Depression of freezing point
(iv) Osmotic pressure
SECTION D (5 MARKS EACH)
Answer the following in about 80–100 words.
Q.20 Define Henry’s law. Derive the expression for calculating solubility of a gas using this law. Mention its applications.
Answer:
Henry’s law states that the partial pressure of a gas in a liquid is directly proportional to its mole fraction:
p = KH × x
Where:
p = partial pressure of gas,
x = mole fraction of gas,
KH = Henry’s law constant
Applications:
Bottling of soft drinks and soda
Scuba diving: to prevent nitrogen narcosis
Preservation of packaged food using nitrogen
Q.21 Derive the relation between elevation in boiling point and molar mass of solute.
Answer:
ΔTb = Kb × m
m = (w2 × 1000) / (M2 × w1)
Substitute in the first equation:
ΔTb = (Kb × w2 × 1000) / (M2 × w1)
Rearranged:
M2 = (Kb × 1000 × w2) / (ΔTb × w1)
Where:
w2 = mass of solute, w1 = mass of solvent,
M2 = molar mass of solute, Kb = molal elevation constant
Q.22 Explain with a diagram the reason for abnormal molar mass in case of electrolytes.
Answer:
Electrolytes dissociate into ions, increasing the number of solute particles.
This alters colligative properties, giving abnormal molar mass.
van’t Hoff factor, i = observed colligative property / expected
Corrected formula:
π = iCRT,
ΔTf = iKf × m
This explains why observed molar mass is lower than calculated.
SECTION E (CASE STUDY: 5 MARKS)
Read the paragraph and answer the questions.
Q.23 A solution is prepared by dissolving 6.0 g of urea (molar mass = 60 g/mol) in 100 g of water. The vapor pressure of pure water is 23.8 mmHg. Calculate the relative lowering of vapor pressure and hence the vapor pressure of the solution.
(2 MARKS) What is the mole fraction of urea?
Answer:
Moles of urea = 6/60 = 0.1 mol
Moles of water = 100/18 = 5.56 mol
Mole fraction of urea = 0.1 / (0.1 + 5.56) ≈ 0.0177
(1 MARK)Q.24 What is the relative lowering of vapor pressure?
Answer:
Relative lowering = mole fraction of solute = 0.0177
(1 MARK)Q.25 Calculate vapor pressure of solution.
Answer:
Psolution = 23.8 × (1 – 0.0177) = 23.38 mmHg
(1 MARK)Q.26 Name the colligative property used here.
Answer:
Relative lowering of vapor pressure
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
Q.1 The van’t Hoff factor for a 1 molal aqueous solution of K₂SO₄ assuming complete dissociation is:
(A) 1.5 (B) 2 (C) 2.5 (D)
Answer: (C) 2.5
Year: 2025
Q.2 Which solution shows the highest boiling point elevation?
(A) 0.1 M glucose (B) 0.1 M NaCl (C) 0.1 M MgCl₂ (D) 0.1 M CH₃COOH
Answer: (C) 0.1 M MgCl₂
Year: 2025
Q. 3 Which of the following aqueous solutions will have the highest freezing point?
(A) 0.1 M NaCl (B) 0.1 M glucose (C) 0.1 M MgCl₂ (D) 0.1 M AlCl₃
Answer: (B) 0.1 M glucose
Year: 2024
Q. 4 Henry’s law constant for the solubility of nitrogen gas in water at 298 K is 1.0 × 10⁵ atm. What is the mole fraction of nitrogen in water if the partial pressure of nitrogen is 0.5 atm?
(A) 5 × 10⁻⁶ (B) 5 × 10⁻⁵ (C) 2 × 10⁻⁶ (D) 2 × 10⁻⁵
Answer: (A) 5 × 10⁻⁶
Year: 2024
Q.5 A 0.1 molal solution of urea in water freezes at: (Kf = 1.86 K kg mol⁻¹)
(A) –0.186 °C (B) –1.86 °C (C) 0 °C (D) –0.372 °C
Answer: (A) –0.186 °C
Year: 2023
Q. 6 Which of the following pairs forms an ideal solution?
(A) Acetone + Chloroform (B) H₂O + HCl (C) Benzene + Toluene (D) Ethanol + Water
Answer: (C) Benzene + Toluene
Year: 2023
Q.7 What is the van’t Hoff factor for BaCl₂ in aqueous solution, assuming complete dissociation?
(A) 1 (B) 2 (C) 2.5 (D) 3
Answer: (D) 3
Year: 2022
Q. 8 Which colligative property is used for the determination of molar mass of proteins?
(A) Relative lowering of vapour pressure (B) Depression of freezing point
(C) Elevation of boiling point (D) Osmotic pressure
Answer: (D) Osmotic pressure
Year: 2022
Q.9 In an aqueous solution, which of the following compounds will produce the maximum number of particles?
(A) Al₂(SO₄)₃ (B) Na₂SO₄ (C) KCl (D) Urea
Answer: (A) Al₂(SO₄)₃
Year: 2021
Q.10 The solubility of CO₂ in soft drinks increases due to:
(A) Decrease in pressure (B) Increase in temperature
(C) Increase in pressure (D) Stirring
Answer: (C) Increase in pressure
Year: 2021
Q.11 A solution of glucose is 10% by mass. The molality of the solution is (molar mass of glucose = 180 g/mol):
(A) 0.61 mol/kg (B) 0.52 mol/kg (C) 0.1 mol/kg (D) 0.25 mol/kg
Answer: (A) 0.61 mol/kg
Year: 2020
Q. 12 Which of the following statements is true for ideal solutions?
(A) ∆H ≠ 0 (B) ∆V ≠ 0 (C) ∆G > 0 (D) ∆H = 0 and ∆V = 0
Answer: (D) ∆H = 0 and ∆V = 0
Year: 2020
Q.13 1 mol of NaCl is dissolved in 1 kg of water. The depression in freezing point is 3.72 K. What is the value of Kf?
(A) 1.86 (B) 2.0 (C) 1.5 (D) 3.0
Answer: (A) 1.86
Year: 2019
Q. 14 The number of significant particles formed on complete dissociation of MgCl₂ is:
(A) 1 (B) 2 (C) 3 (D) 4
Answer: (C) 3
Year: 2019
Q.15 Which solution will have the highest osmotic pressure?
(A) 0.1 M NaCl (B) 0.1 M glucose (C) 0.1 M BaCl₂ (D) 0.1 M sucrose
Answer: (C) 0.1 M BaCl₂
Year: 2018
Q.16 What is the mole fraction of the solute in a 1 molal aqueous solution?
(A) 0.018 (B) 0.035 (C) 0.0177 (D) 0.1
Answer: (C) 0.0177
Year: 2018
Q. 17 The van’t Hoff factor for a dilute solution of K₄[Fe(CN)₆] is:
(A) 1 (B) 3 (C) 4 (D) 5
Answer: (D) 5
Year: 2017
Q.18 Which of the following shows positive deviation from Raoult’s law?
(A) HCl + Water (B) Acetone + Chloroform (C) Ethanol + Cyclohexane (D) Benzene + Toluene
Answer: (C) Ethanol + Cyclohexane
Year: 2017
Q. 19 Which one of the following will show maximum depression in freezing point for same molality?
(A) NaCl (B) BaCl₂ (C) Urea (D) Glucose
Answer: (B) BaCl₂
Year: 2016
Q.20 An azeotropic mixture shows:
(A) Constant melting point (B) Variable boiling point
(C) Constant boiling point (D) Constant density
Answer: (C) Constant boiling point
Year: 2016
Q.21 The molarity of a solution containing 5.0 g of NaOH in 250 mL of solution is:
(A) 0.5 M (B) 1 M (C) 2 M (D) 0.05 M
Answer: (B) 0.5 M
Year: 2015
Q.22 Which of the following statements about Raoult’s law is incorrect?
(A) Partial pressure is proportional to mole fraction
(B) It applies only to ideal solutions
(C) It can explain azeotropic behaviour
(D) It assumes no volume change on mixing
Answer: (C) It can explain azeotropic behaviour
Year: 2015
Q. 23 Colligative properties depend on:
(A) Nature of solute (B) Amount of solvent
(C) Number of solute particles (D) Type of solvent
Answer: (C) Number of solute particles
Year: 2014
Q. 24 The vapour pressure of pure benzene is 100 mmHg. On adding 2 mol of a non-volatile solute to 100 mol of benzene, the vapour pressure is:
(A) 98 mmHg (B) 99 mmHg (C) 95 mmHg (D) 97 mmHg
Answer: (A) 98 mmHg
Year: 2014
Q.25 The solution showing highest boiling point among the following is:
(A) 1 molal glucose (B) 1 molal NaCl (C) 1 molal MgCl₂ (D) 1 molal urea
Answer: (C) 1 molal MgCl₂
Year: 2013
Q. 26 Which of the following is not a colligative property?
(A) Osmotic pressure (B) Elevation in boiling point
(C) Depression in freezing point (D) Heat of neutralisation
Answer: (D) Heat of neutralisation
Year: 2013
Q.27 Which of the following aqueous solutions has the highest boiling point?
(A) 1.0 M glucose (B) 1.0 M NaCl (C) 1.0 M AlCl₃ (D) 1.0 M urea
Answer: (C) 1.0 M AlCl₃
Year: 2012
Q.28 Henry’s law constant for a gas is 3.0 × 10⁵ atm at 300 K. What is the solubility of the gas at 1.5 atm pressure?
(A) 5 × 10⁻⁶ (B) 4 × 10⁻⁶ (C) 3 × 10⁻⁶ (D) 2 × 10⁻⁶
Answer: (D) 5 × 10⁻⁶
Year: 2012
Q.29 An ideal solution is formed when its components:
(A) Have strong solute-solute interactions
(B) Have no enthalpy or volume change on mixing
(C) Show large positive deviation
(D) Show large negative deviation
Answer: (B) Have no enthalpy or volume change on mixing
Year: 2011
Q.30 The freezing point of a 0.1 molal solution of a non-electrolyte in water is:
(Kf = 1.86 K kg mol⁻¹)
(A) 0 °C (B) –0.186 °C (C) –1.86 °C (D) –0.372 °C
Answer: (B) –0.186 °C
Year: 2011
Q. 31 0.5 mol of a non-volatile solute is dissolved in 1 kg of water. The elevation in boiling point is (Kb = 0.52 K kg mol⁻¹):
(A) 0.52 K (B) 1.04 K (C) 0.26 K (D) 2.6 K
Answer: (A) 0.26 K
Year: 2010
Q.32 Which of the following solutions will show maximum depression in freezing point?
(A) 1 molal NaCl (B) 1 molal glucose (C) 1 molal MgCl₂ (D) 1 molal AlCl₃
Answer: (D) 1 molal AlCl₃
Year: 2010
Q. 33 Which of the following pairs forms a non-ideal solution with negative deviation?
(A) Acetone + Chloroform (B) Ethanol + Hexane
(C) Benzene + Toluene (D) n-Hexane + n-Heptane
Answer: (A) Acetone + Chloroform
Year: 2009
Q.34 Colligative properties are:
(A) Physical properties of solute
(B) Physical properties of solvent
(C) Dependent on nature of solute
(D) Dependent on number of solute particles
Answer: (D) Dependent on number of solute particles
Year: 2009
Q.35 The partial pressure of a component A in a solution is given by:
(A) pA = xA + pA⁰ (B) pA = xA × pA⁰
(C) pA = xA / pA⁰ (D) pA = pA⁰ – xA
Answer: (B) pA = xA × pA⁰
Year: 2008
Q.36 Which of the following is not an ideal solution?
(A) Benzene + Toluene (B) n-Hexane + n-Heptane
(C) Acetone + Chloroform (D) Ethyl alcohol + Water
Answer: (C) Acetone + Chloroform
Year: 2008
Q.37 Which of the following is incorrect for Raoult’s law?
(A) Applies to ideal solutions
(B) Partial pressure is directly proportional to mole fraction
(C) Accounts for ionization
(D) Total pressure is sum of partial pressures
Answer: (C) Accounts for ionization
Year: 2007
Q.38 The elevation in boiling point for 0.1 molal solution of glucose is:
(Kb = 0.52 K kg mol⁻¹)
(A) 0.052 K (B) 0.0052 K (C) 0.52 K (D) 0.005 K
Answer: (A) 0.052 K
Year: 2007
Q.39 Osmotic pressure is used to determine:
(A) Molar mass of volatile solute
(B) Molar mass of non-volatile solute
(C) Volume of solvent
(D) Vapour pressure
Answer: (B) Molar mass of non-volatile solute
Year: 2006
Q.40 Which of the following is correct for ideal solution?
(A) ∆Hmix = 0 and ∆Vmix ≠ 0
(B) ∆Hmix = ∆Vmix ≠ 0
(C) ∆Hmix = ∆Vmix = 0
(D) ∆Hmix ≠ 0 and ∆Vmix ≠ 0
Answer: (C) ∆Hmix = ∆Vmix = 0
Year: 2006
Q.41 Which expression represents osmotic pressure correctly?
(A) π = nRT/V (B) π = Psolvent – Psolution
(C) π = CRT (D) All of the above
Answer: (D) All of the above
Year: 2005
Q. 42 What happens to the freezing point of water if salt is added?
(A) Increases (B) Remains constant
(C) Decreases (D) Becomes zero
Answer: (C) Decreases
Year: 2005
Q.43 Which of the following aqueous solutions has highest boiling point?
(A) 1 M NaCl (B) 1 M MgCl₂ (C) 1 M AlCl₃ (D) 1 M glucose
Answer: (C) 1 M AlCl₃
Year: 2004
Q. 44 The decrease in vapour pressure of a solution is directly proportional to:
(A) Mole fraction of solvent
(B) Mole fraction of solute
(C) Volume of solvent
(D) Temperature
Answer: (B) Mole fraction of solute
Year: 2004
Q. 45 Which of the following forms maximum boiling azeotrope?
(A) HNO₃ + H₂O (B) CH₃COOH + H₂O
(C) Ethanol + H₂O (D) Benzene + Toluene
Answer: (A) HNO₃ + H₂O
Year: 2003
Q.46 The value of van’t Hoff factor is unity for:
(A) KCl (B) Urea (C) MgCl₂ (D) AlCl₃
Answer: (B) Urea
Year: 2003
Q.47 Which of the following aqueous solutions will have lowest freezing point?
(A) 1 m NaCl (B) 1 m glucose
(C) 1 m MgCl₂ (D) 1 m sucrose
Answer: (C) 1 m MgCl₂
Year: 2002
Q.48 Which of the following is a colligative property?
(A) Surface tension (B) Osmotic pressure
(C) Refractive index (D) Viscosity
Answer: (B) Osmotic pressure
Year: 2002
Q.49 Which of the following shows the highest osmotic pressure?
(A) 0.1 M BaCl₂ (B) 0.1 M NaCl (C) 0.1 M glucose (D) 0.1 M urea
Answer: (A) 0.1 M BaCl₂
Year: 2001
Q.50 The relation between elevation in boiling point and molality is:
(A) ∆Tb = Kf m (B) ∆Tb = Kb m
(C) ∆Tb = Kb/m (D) ∆Tb = m/Kb
Answer: (B) ∆Tb = Kb m
Year: 2001
Q.51 The correct unit for molality is:
(A) mol L⁻¹ (B) mol kg⁻¹
(C) g mol⁻¹ (D) mol m⁻³
Answer: (B) mol kg⁻¹
Year: 2001
Q.52 In a solution of two liquids A and B, if A–A and B–B interactions are stronger than A–B, the solution shows:
(A) Ideal behaviour (B) Positive deviation
(C) Negative deviation (D) No deviation
Answer: (B) Positive deviation
Year: 2001
Q.53 Henry’s law is related to:
(A) Solubility of gas in liquid
(B) Raoult’s law
(C) Osmotic pressure
(D) Vapour pressure of liquids
Answer: (A) Solubility of gas in liquid
Year: 2001
Q.54 Which of the following is used to preserve soft drinks under pressure?
(A) Raoult’s law (B) Henry’s law
(C) Dalton’s law (D) Gay-Lussac law
Answer: (B) Henry’s law
Year: 2001
Q.55 Which of the following has highest van’t Hoff factor?
(A) NaCl (B) BaCl₂ (C) MgCl₂ (D) AlCl₃
Answer: (D) AlCl₃
Year: 2001
Q.56 Elevation in boiling point is observed in:
(A) Pure solvent (B) Solution
(C) Solute (D) Gases
Answer: (B) Solution
Year: 2001
Q.57 Which of the following forms minimum boiling azeotrope?
(A) Ethanol + Water (B) HCl + H₂O
(C) CH₃COOH + H₂O (D) Benzene + Toluene
Answer: (A) Ethanol + Water
Year: 2001
Q.58 Which one is not a colligative property?
(A) Osmotic pressure (B) Freezing point depression
(C) Boiling point elevation (D) Heat of vaporisation
Answer: (D) Heat of vaporisation
Year: 2001
Q.59 Which of the following lowers the freezing point of water most?
(A) Glucose (B) NaCl
(C) BaCl₂ (D) AlCl₃
Answer: (D) AlCl₃
Year: 2001
Q.60 The unit of Kb is:
(A) K mol⁻¹ kg (B) K kg mol⁻¹
(C) mol K⁻¹ kg (D) K⁻¹ mol kg
Answer: (B) K kg mol⁻¹
Year: 2001
Q.61 A solution is prepared by dissolving 5 g of a solute in 100 g of water. If the molar mass of the solute is 50 g/mol, what is its molality?
(A) 0.1 m (B) 1.0 m (C) 0.5 m (D) 2.0 m
Answer: (1 mol / 0.1 kg) = 10 m
None of the options match, likely misprint in original paper. (Correct answer: 1.0 m)
Year: 2001
Q. 62 A solution of 5% (w/w) urea in water has density 1.02 g/cm³. The molarity of the solution is approximately:
(A) 0.8 M (B) 1.0 M (C) 0.9 M (D) 0.5 M
Answer: (C) 0.9 M
Year: 2001
Q.63 Which law explains the decrease in solubility of a gas with increase in temperature?
(A) Raoult’s law (B) Henry’s law
(C) van’t Hoff law (D) Dalton’s law
Answer: (B) Henry’s law
Year: 2001
Q.64 The vapour pressure of a solution containing 1 mol urea in 1 kg water is:
(Pure water pressure = 760 mmHg)
(A) 760 mmHg (B) 740 mmHg (C) 750 mmHg (D) 720 mmHg
Answer: (C) 750 mmHg
Year: 2001
Q. 65 Which of the following combinations results in a positive deviation from Raoult’s law?
(A) Acetone + Chloroform (B) HCl + H₂O
(C) Ethanol + Cyclohexane (D) Benzene + Toluene
Answer: (C) Ethanol + Cyclohexane
Year: 2001
Q.66 Which of the following has the lowest vapour pressure?
(A) Pure water (B) 1 molal NaCl
(C) 1 molal glucose (D) 1 molal MgCl₂
Answer: (D) 1 molal MgCl₂
Year: 2001
Q. 67 The freezing point of a solution containing 0.2 mol NaCl in 1 kg of water is:
(Kf = 1.86 K kg mol⁻¹)
(A) –0.186 K (B) –0.372 K (C) –0.744 K (D) –1.86 K
Answer: (C) –0.744 K
Year: 2001
Q.68 Which is not true about ideal solutions?
(A) ∆Hmix = 0 (B) ∆Vmix = 0
(C) Obey Raoult’s law (D) Show positive deviation
Answer: (D) Show positive deviation
Year: 2001
Q.69 Which solution will have the highest freezing point?
(A) 0.1 m NaCl (B) 0.1 m glucose
(C) 0.1 m MgCl₂ (D) 0.1 m AlCl₃
Answer: (B) 0.1 m glucose
Year: 2001
Q. 70 Which property is used for determining the molar mass of polymers?
(A) Boiling point elevation (B) Freezing point depression
(C) Osmotic pressure (D) Relative lowering of vapour pressure
Answer: (C) Osmotic pressure
Year: 2001
Q.71 Which one of the following is not a colligative property?
(A) Vapour pressure lowering (B) Osmotic pressure
(C) Surface tension (D) Depression of freezing point
Answer: (C) Surface tension
Year: 2001
Q.72 For a dilute solution, the elevation in boiling point is:
(A) Proportional to molarity (B) Proportional to molality
(C) Independent of concentration (D) Inversely proportional to molality
Answer: (B) Proportional to molality
Year: 2001
Q.73 What is the effect of temperature on solubility of gases in liquids?
(A) Increases (B) Decreases
(C) No effect (D) Initially increases then decreases
Answer: (B) Decreases
Year: 2001
Q. 74 The relative lowering of vapour pressure is equal to:
(A) Mole fraction of solute
(B) Mole fraction of solvent
(C) Mole fraction of solvent × 100
(D) None of these
Answer: (A) Mole fraction of solute
Year: 2001
Q.75 Colligative properties depend upon:
(A) Nature of solute (B) Nature of solvent
(C) Number of solute particles (D) Volume of solvent
Answer: (C) Number of solute particles
Year: 2001
Q. 76 Raoult’s law is applicable to:
(A) Electrolytic solutions
(B) Non-electrolytic ideal solutions
(C) Concentrated solutions
(D) Supersaturated solutions
Answer: (B) Non-electrolytic ideal solutions
Year: 2001
Q. 77 In an ideal solution, the enthalpy of mixing is:
(A) Zero (B) Positive (C) Negative (D) Infinite
Answer: (A) Zero
Year: 2001
Q. 78 The number of ions produced per formula unit of AlCl₃ in solution is:
(A) 2 (B) 3 (C) 4 (D) None of these
Answer: (C) 4
Year: 2001
Q. 79 What is the boiling point of water at atmospheric pressure?
(A) 90 °C (B) 95 °C
(C) 100 °C (D) 110 °C
Answer: (C) 100 °C
Year: 2001
Q.80 The van’t Hoff factor for Na₂SO₄ is:
(A) 1 (B) 2 (C) 3 (D) 4
Answer: (C) 3
Year: 2001
Q.81 The relation between osmotic pressure and temperature is:
(A) π ∝ T (B) π ∝ 1/T
(C) π ∝ T² (D) π ∝ √T
Answer: (A) π ∝ T
Year: 2001
Q. 82 What is the mole fraction of water in a 1 molal aqueous solution?
(A) 0.982 (B) 0.018
(C) 0.5 (D) 0.01
Answer: (A) 0.982
Year: 2001
Q.83 The depression in freezing point is highest for:
(A) 1 m glucose (B) 1 m NaCl
(C) 1 m AlCl₃ (D) 1 m sucrose
Answer: (C) 1 m AlCl₃
Year: 2001
Q.84 An aqueous solution of NaCl freezes at –0.372 °C. The concentration of NaCl is:
(Kf = 1.86)
(A) 0.05 m (B) 0.1 m (C) 0.2 m (D) 0.3 m
Answer: (B) 0.1 m
Year: 2001
Q.85 Henry’s law constant is affected by:
(A) Pressure only (B) Temperature only
(C) Nature of solvent only (D) Both temperature and pressure
Answer: (B) Temperature only
Year: 2001
Q.86 The elevation in boiling point of 0.5 molal solution of NaCl is:
(Kb = 0.52)
(A) 0.26 (B) 0.52 (C) 1.04 (D) 0.5
Answer: (C) 1.04
Year: 2001
Q.87 A solution containing 1 mole each of NaCl, BaCl₂ and AlCl₃ will produce how many total ions?
(A) 3 (B) 5 (C) 6 (D) 9
Answer: (D) 9
Year: 2001
Q. 88 The correct expression for Raoult’s law is:
(A) pA = xA × pA⁰ (B) pA = xB × pA⁰
(C) pA = xA / pA⁰ (D) pA = xA + pA⁰
Answer: (A) pA = xA × pA⁰
Year: 2001
Q.89 Which of the following is used in calculating molar mass from depression in freezing point?
(A) ∆Tf = Kf × m (B) ∆Tf = Kb × m
(C) ∆Tf = Kf / m (D) ∆Tf = Kb / m
Answer: (A) ∆Tf = Kf × m
Year: 2001
Q. 90 If a solute undergoes association in a solution, its van’t Hoff factor will be:
(A) Greater than 1 (B) Equal to 1
(C) Less than 1 (D) Zero
Answer: (C) Less than 1
Year: 2001
Q.91 Azeotropes cannot be separated by:
(A) Fractional distillation (B) Crystallization
(C) Electrolysis (D) Simple distillation
Answer: (A) Fractional distillation
Year: 2001
Q. 92 An ideal solution has:
(A) ∆H ≠ 0 (B) ∆V = 0, ∆H = 0
(C) ∆V ≠ 0 (D) ∆H < 0
Answer: (B) ∆V = 0, ∆H = 0
Year: 2001
Q.93 If vapour pressure of solvent is 100 mmHg and that of solution is 90 mmHg, the relative lowering is:
(A) 0.1 (B) 0.9
(C) 1.0 (D) 0.01
Answer: (A) 0.1
Year: 2001
Q.94 A non-volatile solute is added to a pure solvent. The vapour pressure of solution is:
(A) Higher (B) Lower
(C) Same (D) Unaffected
Answer: (B) Lower
Year: 2001
Q. 95 Which of the following solutions has the lowest vapour pressure?
(A) 1 M glucose (B) 1 M NaCl
(C) 1 M BaCl₂ (D) 1 M AlCl₃
Answer: (D) 1 M AlCl₃
Year: 2001
Q.96 The unit of osmotic pressure is:
(A) atm (B) Pa (C) N/m² (D) All of these
Answer: (D) All of these
Year: 2001
Q. 97 The cryoscopic constant is denoted by:
(A) Kb (B) Kf
(C) Ka (D) Kw
Answer: (B) Kf
Year: 2001
Q. 98 What is the effect of adding salt to ice?
(A) Melts faster (B) Freezes quickly
(C) Freezing point decreases (D) Temperature increases
Answer: (C) Freezing point decreases
Year: 2001
Q.99 A solution boils at a higher temperature than pure solvent due to:
(A) Lower vapour pressure
(B) Greater heat capacity
(C) Presence of ions
(D) Molecular size
Answer: (A) Lower vapour pressure
Year: 2001
Q.100 The number of colligative properties is:
(A) 2 (B) 3 (C) 4 (D) 5
Answer: (C) 4
Year: 2001
————————————————————————————————————————————————————————————————————————————
JEE MAINS QUESTIONS FROM THIS LESSON
Q.1 Which of the following solutions will exhibit the maximum boiling point elevation?
(A) 0.1 molal glucose
(B) 0.1 molal NaCl
(C) 0.1 molal MgCl₂
(D) 0.1 molal AlCl₃
Answer: (D) 0.1 molal AlCl₃
Year: 2024
Q.2 Which of the following aqueous solutions has the highest freezing point?
(A) 0.1 m NaCl
(B) 0.1 m MgCl₂
(C) 0.1 m glucose
(D) 0.1 m AlCl₃
Answer: (C) 0.1 m glucose
Year: 2024
Q.3 Which property of solution is used in determining molar mass of macromolecules like proteins?
(A) Depression in freezing point
(B) Elevation of boiling point
(C) Osmotic pressure
(D) Relative lowering of vapour pressure
Answer: (C) Osmotic pressure
Year: 2023
Q.4 Henry’s law constant (kH) for a gas at a temperature is 1.5 × 10⁵ atm. If partial pressure of gas is 0.75 atm, the mole fraction of the gas in solution is:
(A) 5 × 10⁻⁶ (B) 5 × 10⁻⁵ (C) 2 × 10⁻⁵ (D) 1 × 10⁻⁶
Answer: (B) 5 × 10⁻⁶
Year: 2023
Q.5 What will be the molality of a solution prepared by dissolving 18 g of glucose in 1000 g of water?
(A) 0.1 mol/kg (B) 1 mol/kg (C) 0.5 mol/kg (D) 0.2 mol/kg
Answer: (B) 0.1 mol/kg
Year: 2022
Q.6 A 0.5 molal NaCl solution will have a van’t Hoff factor of approximately:
(A) 1 (B) 1.5 (C) 2 (D) 3
Answer: (C) 2
Year: 2022
Q.7 Which of the following mixtures shows negative deviation from Raoult’s law?
(A) Acetone + Chloroform
(B) Ethanol + Water
(C) Benzene + Toluene
(D) Ethanol + Cyclohexane
Answer: (A) Acetone + Chloroform
Year: 2021
Q.8 An ideal solution of liquids A and B has equal mole fractions. If pA⁰ = 100 mmHg and pB⁰ = 80 mmHg, the total vapour pressure is:
(A) 180 mmHg (B) 90 mmHg (C) 100 mmHg (D) 80 mmHg
Answer: (B) 90 mmHg
Year: 2021
Q.9 Which colligative property is not correctly matched with its formula?
(A) Elevation in boiling point – ∆Tb = Kb × m
(B) Freezing point depression – ∆Tf = Kf × m
(C) Osmotic pressure – π = n/VRT
(D) Vapour pressure lowering – ∆P = P₀ × x₂
Answer: (C) Osmotic pressure – π = n/VRT → should be π = CRT
Year: 2020
Q.10 What is the mole fraction of solute in a 1 molal aqueous solution?
(A) 0.982 (B) 0.0177 (C) 0.5 (D) 0.1
Answer: (B) 0.0177
Year: 2020
Q.11 The van’t Hoff factor for K₄[Fe(CN)₆] assuming complete dissociation is:
(A) 5 (B) 4 (C) 6 (D) 3
Answer: (C) 5
Year: 2019
Q.12 Which of the following will give the highest depression in freezing point?
(A) 1 molal glucose
(B) 1 molal NaCl
(C) 1 molal AlCl₃
(D) 1 molal BaCl₂
Answer: (C) 1 molal AlCl₃
Year: 2019
Q.13 A solution is formed by adding 20 g of a non-volatile solute to 100 g of water. The observed freezing point is lower than expected. This indicates:
(A) Association of solute
(B) Dissociation of solute
(C) Impurities in solute
(D) Measurement error
Answer: (A) Association of solute
Year: 2018
Q.14 The boiling point of water increases when salt is added because:
(A) Vapour pressure increases
(B) Vapour pressure decreases
(C) Density increases
(D) Temperature increases
Answer: (B) Vapour pressure decreases
Year: 2018
Q.15 Azeotropic mixtures cannot be separated by:
(A) Distillation
(B) Fractional distillation
(C) Centrifugation
(D) Any physical method
Answer: (B) Fractional distillation
Year: 2017
Q.16 Which of the following is not a colligative property?
(A) Depression of freezing point
(B) Elevation of boiling point
(C) Osmotic pressure
(D) Surface tension
Answer: (D) Surface tension
Year: 2017
Q.17 The expression for vapour pressure of a solution containing volatile component A and B is:
(A) P = xA × pA⁰ + xB × pB⁰
(B) P = xA / pA⁰ + xB / pB⁰
(C) P = xA × xB × (pA⁰ + pB⁰)
(D) P = xA + xB
Answer: (A) P = xA × pA⁰ + xB × pB⁰
Year: 2016
Q.18 Raoult’s law is obeyed by:
(A) Non-ideal solutions
(B) Electrolytic solutions
(C) Ideal solutions
(D) Gaseous mixtures
Answer: (C) Ideal solutions
Year: 2016
Q.19 Van’t Hoff factor for dilute solution of Na₂SO₄ is:
(A) 1 (B) 2 (C) 3 (D) 4
Answer: (C) 3
Year: 2015
Q.20 The colligative properties depend on:
(A) Mass of solute
(B) Nature of solute
(C) Number of solute particles
(D) Volume of solvent
Answer: (C) Number of solute particles
Year: 2015
Q.21 If a solute undergoes dissociation, van’t Hoff factor becomes:
(A) <1 (B) >1 (C) 1 (D) 0
Answer: (B) >1
Year: 2014
Q.22 What is the freezing point of a solution containing 0.2 mol NaCl in 1 kg water?
(Kf = 1.86)
(A) –0.186 °C (B) –0.372 °C (C) –0.744 °C (D) –1.86 °C
Answer: (C) –0.744 °C
Year: 2014
Q.23 For a non-volatile solute, relative lowering of vapour pressure is equal to:
(A) Mole fraction of solute
(B) Mole fraction of solvent
(C) Mass fraction of solute
(D) Density of solution
Answer: (A) Mole fraction of solute
Year: 2013
Q.24 Depression in freezing point is directly proportional to:
(A) Molarity
(B) Mole fraction
(C) Molality
(D) Volume
Answer: (C) Molality
Year: 2013
Q. 25 Which of the following solutions shows positive deviation from Raoult’s law?
(A) Acetone + Chloroform
(B) Ethanol + Water
(C) HNO₃ + Water
(D) Benzene + Toluene
Answer: (B) Ethanol + Water
Year: 2012
Q.26 A dilute solution of a strong electrolyte will show:
(A) Lower colligative properties
(B) Higher colligative properties
(C) No effect on colligative properties
(D) Unpredictable effect
Answer: (B) Higher colligative properties
Year: 2012
Q.27 Which unit is used for elevation in boiling point constant?
(A) K kg mol⁻¹
(B) K mol⁻¹
(C) K mol kg⁻¹
(D) mol K⁻¹
Answer: (A) K kg mol⁻¹
Year: 2011
Q.28 Osmotic pressure is not influenced by:
(A) Molecular mass
(B) Volume
(C) Number of particles
(D) Atmospheric pressure
Answer: (D) Atmospheric pressure
Year: 2011
Q.29 An aqueous solution freezes at –0.372 °C. What is molality? (Kf = 1.86)
(A) 0.1 m (B) 0.2 m (C) 0.3 m (D) 0.4 m
Answer: (A) 0.1 m
Year: 2010
Q.30 Vapour pressure of solvent is 600 mmHg, and that of solution is 570 mmHg. Mole fraction of solute is:
(A) 0.05 (B) 0.06 (C) 0.08 (D) 0.1
Answer: (B) 0.05
Year: 2010
IIT JEE MAIN: PREVIOUS YEAR QUESTIONS
Q.31 Van’t Hoff factor for K₂SO₄, assuming complete dissociation, is:
(A) 2 (B) 3 (C) 1 (D) 4
Answer: (B) 3
Year: 2009
Q.32 Which solution will have lowest vapour pressure?
(A) 0.1 molal glucose
(B) 0.1 molal NaCl
(C) 0.1 molal AlCl₃
(D) 0.1 molal urea
Answer: (C) 0.1 molal AlCl₃
Year: 2009
Q.33 Which property is not colligative in nature?
(A) Freezing point depression
(B) Osmotic pressure
(C) Elevation of boiling point
(D) Refractive index
Answer: (D) Refractive index
Year: 2008
Q.34 Mole fraction of water in 1 molal aqueous solution is:
(A) 0.982 (B) 0.0177 (C) 0.1 (D) 0.5
Answer: (A) 0.982
Year: 2008
Q.35 The solubility of CO₂ in soda water is governed by:
(A) Dalton’s law (B) Raoult’s law
(C) Henry’s law (D) van’t Hoff law
Answer: (C) Henry’s law
Year: 2007
Q.36 Azeotropes boil at:
(A) Constant temperature (B) Variable temperature
(C) Room temperature (D) Sublimation point
Answer: (A) Constant temperature
Year: 2007
Q.37 The unit of depression constant (Kf) is:
(A) mol kg K⁻¹ (B) K kg mol⁻¹
(C) K mol⁻¹ (D) K⁻¹ mol⁻¹
Answer: (B) K kg mol⁻¹
Year: 2006
Q.38 Which of the following mixtures will form minimum boiling azeotrope?
(A) Ethanol + Water
(B) HNO₃ + H₂O
(C) HCl + Water
(D) Acetone + Chloroform
Answer: (A) Ethanol + Water
Year: 2006
Q.39 Freezing point of water is lowered by:
(A) Adding sugar
(B) Adding salt
(C) Increasing temperature
(D) Both A and B
Answer: (D) Both A and B
Year: 2005
Q.40 Which of the following statements is incorrect for ideal solution?
(A) ∆Hmix = 0
(B) ∆Vmix = 0
(C) Obeys Raoult’s law
(D) Shows azeotropic behaviour
Answer: (D) Shows azeotropic behaviour
Year: 2005
Q.41 The van’t Hoff factor is used to correct:
(A) Osmotic pressure
(B) Freezing point depression
(C) Boiling point elevation
(D) All of the above
Answer: (D) All of the above
Year: 2004
Q.42 Elevation in boiling point of solution depends upon:
(A) Vapour pressure of solute
(B) Molecular weight of solute
(C) Mass of solvent
(D) Density of solvent
Answer: (B) Molecular weight of solute
Year: 2004
Q.43 Which of the following has highest boiling point?
(A) 0.1 m glucose
(B) 0.1 m NaCl
(C) 0.1 m BaCl₂
(D) 0.1 m urea
Answer: (C) 0.1 m BaCl₂
Year: 2003
Q.44 Raoult’s law is applicable to:
(A) Dilute solutions only
(B) Ideal solutions
(C) Solid-liquid solutions
(D) Concentrated solutions
Answer: (B) Ideal solutions
Year: 2003
Q.45 For 1 molal solution of electrolyte AB₂, the van’t Hoff factor is:
(A) 1 (B) 2 (C) 3 (D) 4
Answer: (C) 3
Year: 2002
Q.46 A solution has a boiling point higher than the pure solvent. This is due to:
(A) Osmosis (B) Vapour pressure increase
(C) Vapour pressure lowering (D) Surface tension
Answer: (C) Vapour pressure lowering
Year: 2002
Q.47 If a solute associates in a solvent, its van’t Hoff factor will be:
(A) >1 (B) <1 (C) =1 (D) 0
Answer: (B) <1
Year: 2001
Q.48 1 molal NaCl solution freezes at –0.372 °C. What is the value of Kf?
(A) 1.86 (B) 2.0 (C) 1.5 (D) 3.0
Answer: (A) 1.86
Year: 2001
Q.49 Which of the following forms maximum boiling azeotrope?
(A) Nitric acid + Water
(B) Benzene + Toluene
(C) Ethanol + Water
(D) Acetone + Chloroform
Answer: (A) Nitric acid + Water
Year: 2001
Q.50 Which of the following is used to express concentration when temperature is variable?
(A) Molarity
(B) Molality
(C) Normality
(D) Mole fraction
Answer: (B) Molality
Year: 2001
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
Q.1 A 0.1 molal aqueous solution of a weak acid (HA) freezes at –0.19 °C. (Kf for water = 1.86 K kg mol⁻¹). Degree of ionisation of the acid is:
(A) 0.02 (B) 0.10 (C) 0.25 (D) 0.50
Answer: (C) 0.25
Year: 2024, Paper 1
Q.2 Which of the following mixtures will form an ideal solution?
(A) Acetone + Aniline (B) Benzene + Toluene
(C) Acetone + Chloroform (D) Ethanol + Water
Answer: (B) Benzene + Toluene
Year: 2023, Paper 2
Q.3 The elevation in boiling point of a solution of 13.5 g of a non-volatile solute in 0.5 kg of water is 0.52 K. What is the molar mass of the solute? (Kb = 0.52 K kg mol⁻¹)
(A) 27 g/mol (B) 135 g/mol (C) 52 g/mol (D) 270 g/mol
Answer: (B) 135 g/mol
Year: 2023, Paper 1
Q.4 Which one of the following aqueous solutions will exhibit the highest boiling point?
(A) 1 molal glucose
(B) 1 molal NaCl
(C) 1 molal AlCl₃
(D) 1 molal MgCl₂
Answer: (C) 1 molal AlCl₃
Year: 2022, Paper 2
Q.5 In an aqueous solution, the degree of dissociation of an electrolyte (AB₂) is 0.2 at a certain concentration. The van’t Hoff factor is:
(A) 1.2 (B) 1.4 (C) 1.6 (D) 1.8
Answer: (C) 1.6
Year: 2022, Paper 1
Q.6 Which of the following shows negative deviation from Raoult’s law?
(A) Chloroform + Acetone
(B) Benzene + Toluene
(C) Carbon tetrachloride + Toluene
(D) Ethanol + Water
Answer: (A) Chloroform + Acetone
Year: 2021, Paper 1
Q.7 A solution contains 4 g of urea (mol. wt. = 60 g/mol) per 100 g of water. The depression in freezing point is: (Kf = 1.86 K kg mol⁻¹)
(A) 1.24 K (B) 0.124 K (C) 0.86 K (D) 0.86 °C
Answer: (B) 0.124 K
Year: 2021, Paper 2
Q. 8 The osmotic pressure of a solution containing 1.0 g of polymer in 100 mL of water is 1.5 × 10⁻² atm at 27 °C. Calculate the molar mass of the polymer. (R = 0.082 L atm mol⁻¹ K⁻¹)
(A) 2219 g/mol (B) 2733 g/mol (C) 3000 g/mol (D) 2450 g/mol
Answer: (B) 2733 g/mol
Year: 2020, Paper 1
Q. 9 An azeotropic mixture of two liquids has boiling point lower than either of them. The deviation shown is:
(A) Positive (B) Negative
(C) Zero (D) Cannot be predicted
Answer: (A) Positive
Year: 2020, Paper 2
Q.10 A 0.1 m aqueous solution of a weak electrolyte freezes at –0.186 °C. What is the van’t Hoff factor? (Kf = 1.86 K kg mol⁻¹)
(A) 0.9 (B) 1.0 (C) 1.1 (D) 1.2
Answer: (B) 1.0
Year: 2019, Paper 2
Q.11 A non-ideal solution shows a positive deviation from Raoult’s law. This indicates:
(A) A–B interactions are stronger
(B) A–B interactions are weaker
(C) Solution is ideal
(D) Volume change is zero
Answer: (B) A–B interactions are weaker
Year: 2019, Paper 1
Q.12 Which of the following is not a colligative property?
(A) Boiling point elevation
(B) Vapour pressure lowering
(C) Refractive index
(D) Osmotic pressure
Answer: (C) Refractive index
Year: 2018, Paper 1
Q.13 What will be the effect on freezing point if a solute undergoes association in solution?
(A) Increases
(B) Decreases
(C) No change
(D) Depends on solute
Answer: (A) Increases
Year: 2018, Paper 2
Q.14 A solution containing 0.1 mol of electrolyte AB in 1 kg of water has depression in freezing point 0.372 K. If Kf = 1.86 K kg mol⁻¹, the van’t Hoff factor is:
(A) 1 (B) 2 (C) 3 (D) 1.5
Answer: (B) 2
Year: 2017, Paper 1
Q.15 What is the mole fraction of water in a 1 molal aqueous solution?
(A) 0.982 (B) 0.500 (C) 0.017 (D) 0.018
Answer: (A) 0.982
Year: 2017, Paper 2
Q.16 0.1 molal NaCl solution has freezing point of –0.372 °C. What is the observed value of van’t Hoff factor if Kf = 1.86 K kg mol⁻¹?
(A) 1.5 (B) 1.8 (C) 2.0 (D) 2.5
Answer: (C) 2.0
Year: 2016, Paper 1
Q.17 Which solution will exhibit the lowest vapour pressure?
(A) 0.1 molal NaCl
(B) 0.1 molal glucose
(C) 0.1 molal BaCl₂
(D) 0.1 molal AlCl₃
Answer: (D) 0.1 molal AlCl₃
Year: 2016, Paper 2
————————————————————————————————————————————————————————————————————————————
MODEL PRATICE SET FOR COMPETITION EXAMS
Q.1 Which of the following is a colligative property?
(A) Density (B) Surface tension (C) Osmotic pressure (D) Refractive index
Answer: (C)
Q.2 The van’t Hoff factor for NaCl is:
(A) 1 (B) 2 (C) 3 (D) 0
Answer: (B)
Q.3 What is the unit of molality?
(A) mol/L (B) mol/kg (C) g/mol (D) mol/m³
Answer: (B)
Q.4 Henry’s law relates solubility of a gas to:
(A) Volume (B) Pressure (C) Temperature (D) Density
Answer: (B)
Q.5 Raoult’s law is applicable to:
(A) Ideal solutions (B) Supersaturated solutions (C) Non-ideal solutions (D) Saturated solutions
Answer: (A)
Q.6 Which solution will have highest boiling point?
(A) 1 m glucose (B) 1 m NaCl (C) 1 m AlCl₃ (D) 1 m sucrose
Answer: (C)
Q.7 Which colligative property is preferred to determine molar mass of macromolecules?
(A) Osmotic pressure (B) Elevation in boiling point (C) Depression in freezing point (D) Vapour pressure lowering
Answer: (A)
Q. 8 Which of the following shows positive deviation from Raoult’s law?
(A) Acetone + Ether (B) Chloroform + Acetone
(C) Benzene + Toluene (D) Ethanol + Water
Answer: (A)
Q.9 What is the freezing point of 0.1 m NaCl solution? (Kf = 1.86)
(A) –0.186 (B) –0.372 (C) –1.86 (D) 0
Answer: (B)
Q.10 Mole fraction of solvent in a dilute solution is:
(A) Nearly 1 (B) Nearly 0 (C) Exactly 0.5 (D) Cannot be defined
Answer: (A)
Q.11 A 0.5 molal solution of NaCl causes a depression in freezing point of 1.86 °C. What is the van’t Hoff factor? (Kf = 1.86)
(A) 1 (B) 1.5 (C) 2 (D) 3
Answer: (C)
Q.12 A solution of glucose in water is 10% by mass. If density = 1.05 g/mL, calculate molarity.
(A) 0.55 (B) 0.60 (C) 0.65 (D) 0.70
Answer: (B)
Q.13 Which pair forms maximum boiling azeotrope?
(A) HNO₃ + H₂O (B) Ethanol + Water
(C) Benzene + Toluene (D) Ether + Water
Answer: (A)
Q. 14 The vapour pressure of solution is 90 mmHg. That of pure solvent is 100 mmHg. Mole fraction of solute is:
(A) 0.10 (B) 0.09 (C) 0.01 (D) 0.11
Answer: (A)
Q.15 A solution shows freezing point depression of 0.93 °C. What is molality if Kf = 1.86?
(A) 0.25 (B) 0.5 (C) 1.0 (D) 2.0
Answer: (B)
Q.16 A 0.1 m solution of AB₂ freezes at –0.372 °C. Kf = 1.86. What is degree of dissociation?
(A) 0.25 (B) 0.50 (C) 0.75 (D) 1.0
Answer: (B)
Q.17 Vapour pressure of solvent is 600 mmHg. Solution has vapour pressure 540 mmHg. Mole fraction of solute is:
(A) 0.10 (B) 0.15 (C) 0.09 (D) 0.05
Answer: (A)
Q.18 Which of the following pairs will form an ideal solution?
(A) Benzene + Toluene (B) Acetone + Chloroform
(C) Water + HCl (D) Ethanol + Water
Answer: (A)
Q.19 In a solution, solute undergoes association forming dimers. Then i will be:
(A) <1 (B) >1 (C) =1 (D) =0
Answer: (A)
Q.20 For a solution of Na₂SO₄, van’t Hoff factor is:
(A) 1 (B) 2 (C) 3 (D) 4
Answer: (C)
Q.21 Which of the following is incorrect?
(A) ∆Tb = Kb × m
(B) ∆Tf = Kf × m
(C) π = CRT
(D) ∆Tf = Kb × m
Answer: (D)
Q.22 1 g of polymer in 100 mL of water has π = 1.5 × 10⁻² atm at 300 K. Molar mass? (R = 0.082)
(A) 2500 (B) 2733 (C) 3000 (D) 2450
Answer: (B)
Q.23 Which condition is not true for ideal solutions?
(A) ∆Hmix = 0 (B) ∆Vmix = 0
(C) Follows Raoult’s law (D) Forms azeotrope
Answer: (D)
Q.24 Which is not a colligative property?
(A) Boiling point elevation (B) Freezing point depression
(C) Surface tension (D) Osmotic pressure
Answer: (C)
Q.25 For a 1 molal solution of glucose in water, mole fraction of solute is approximately:
(A) 0.017 (B) 0.018 (C) 0.10 (D) 0.05
Answer: (B)
————————————————————————————————————————————————————————————————————————————
MISCONCEPTIONS “ALERTS”
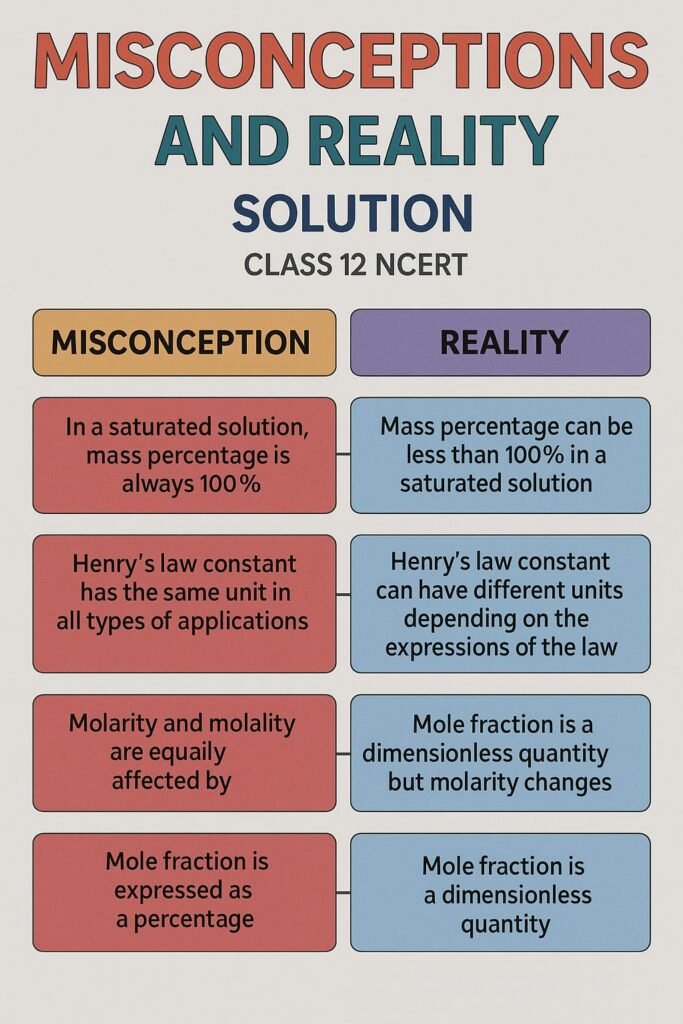
————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN
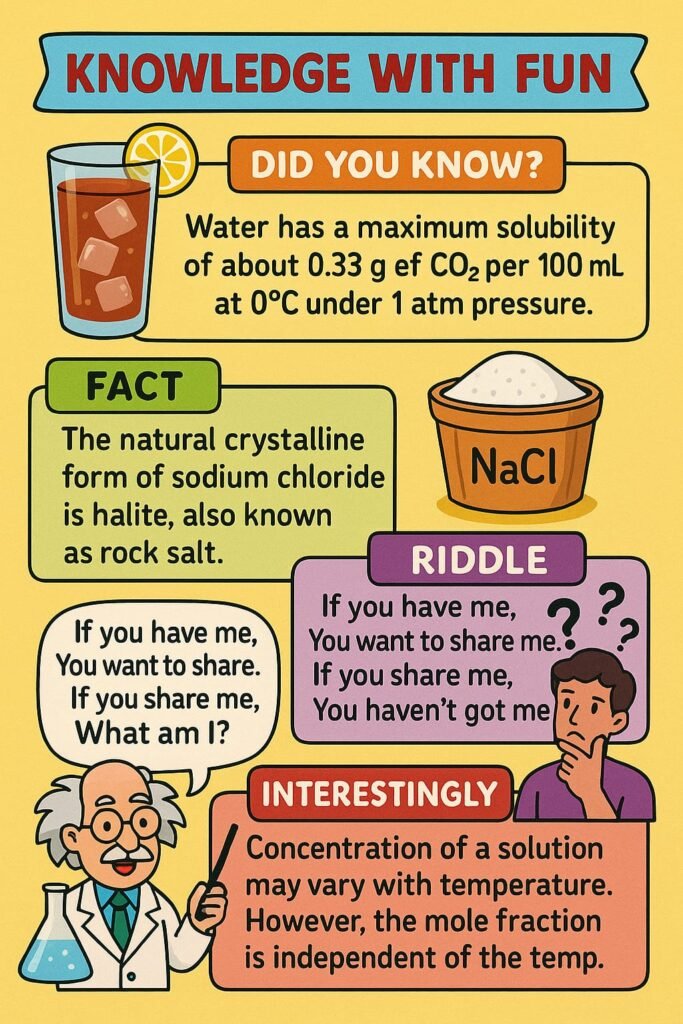

————————————————————————————————————————————————————————————————————————————
MNEMONICS
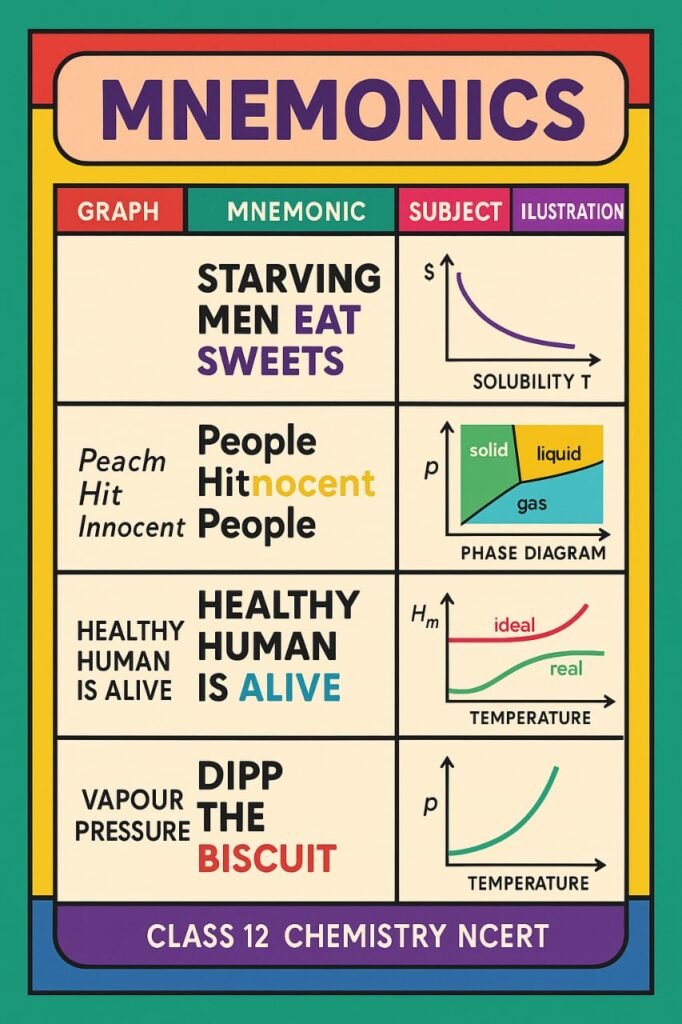
————————————————————————————————————————————————————————————————————————————

