Class 11 : Chemistry (In English) – Chapter 6: Equilibrium
EXPLANATION & SUMMARY
🔵 Introduction
Chemical reactions don’t always go to completion 🔁. Some stop midway, giving a mixture of reactants and products. This balance is called equilibrium ⚖. Equilibrium is central to chemistry — it explains how reactions occur in reversible ways, why some reactions yield little product, and how conditions like pressure and temperature affect the outcome.
Equilibrium is studied in two parts:
Physical Equilibrium → changes of state (solid–liquid, liquid–gas, etc.).
Chemical Equilibrium → reversible reactions in chemistry.
🟢 Equilibrium in Physical Processes
Examples:
Ice ⇌ Water (solid–liquid equilibrium).
H₂O(l) ⇌ H₂O(g) (liquid–vapor equilibrium).
Solute ⇌ Solution (saturated solution equilibrium).
Characteristics:
✔ Dynamic (forward and backward processes occur simultaneously).
✔ Rate of forward = rate of backward reaction.
✔ Observable properties constant (pressure, concentration).
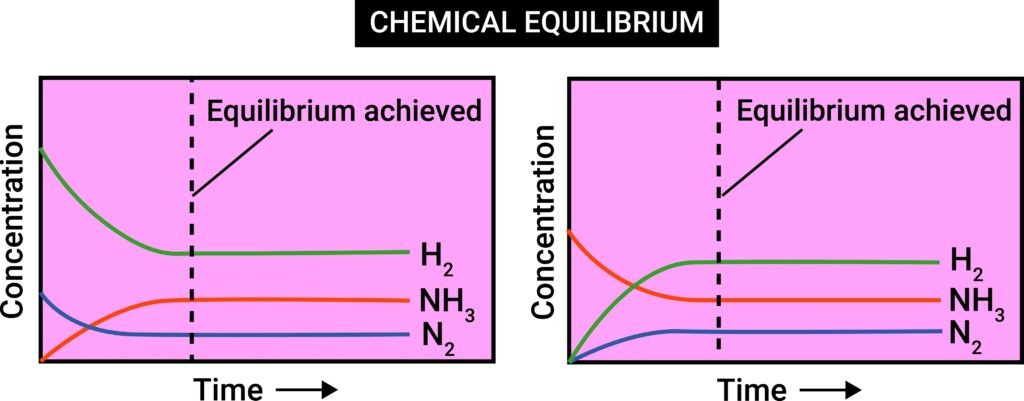
🔵 Equilibrium in Chemical Processes
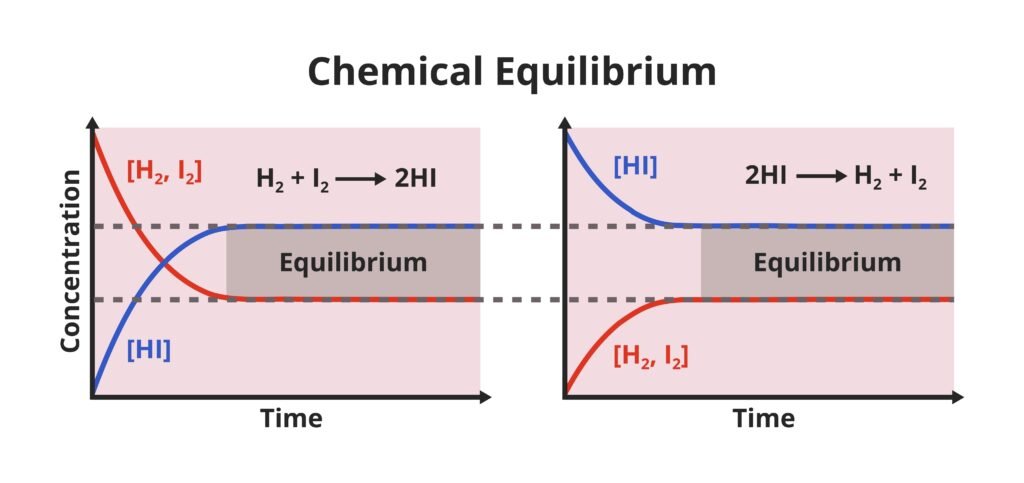
Many chemical reactions are reversible. Example:
N₂ + 3H₂ ⇌ 2NH₃
When forward reaction equals backward reaction → chemical equilibrium.
📌 Characteristics:
Dynamic state.
Reached only in closed system.
Concentrations constant at equilibrium.
Equilibrium state depends on temperature, pressure, concentration.
🟡 Law of Mass Action (Guldberg & Waage, 1864)
For a general reaction:
aA + bB ⇌ cC + dD
Rate ∝ [reactants]^coefficients.
At equilibrium,
Kc = [C]^c [D]^d / [A]^a [B]^b
This is the equilibrium constant (Kc).
🔴 Equilibrium Constant
Two forms:
Kc (concentration-based).
Kp (pressure-based, for gases).
Relation between Kp and Kc:
Kp = Kc (RT)^Δn
where Δn = (moles of gaseous products − moles of gaseous reactants).
🟢 Significance of K
If K is large (>>1): equilibrium lies towards products.
If K is small (<<1): equilibrium lies towards reactants.
If K ≈ 1: appreciable concentrations of both reactants and products.
🔵 Reaction Quotient (Q)
Same as K expression but for initial concentrations.
If Q < K → forward reaction dominates.
If Q > K → backward reaction dominates.
If Q = K → equilibrium attained.
🟡 Le Chatelier’s Principle (1884)
📌 If a system at equilibrium is disturbed, it shifts to counteract the disturbance.
Change in Concentration
Increase reactants → shift forward.
Increase products → shift backward.
Change in Pressure (for gases)
Increase pressure → shift towards fewer moles of gas.
Decrease pressure → shift towards more moles.
Change in Temperature
Exothermic reaction (ΔH < 0): increase T → shift backward.
Endothermic reaction (ΔH > 0): increase T → shift forward.
✔ Applications: Haber process, Contact process, industrial synthesis.
🔴 Ionic Equilibrium in Aqueous Solutions
When ionic compounds dissolve, they dissociate into ions. The equilibria involving ions are crucial.
1️⃣ Electrolytes
Strong electrolytes: completely ionize (NaCl, HCl).
Weak electrolytes: partially ionize (CH₃COOH, NH₃).
2️⃣ Ionization Equilibrium of Weak Electrolytes
Example: CH₃COOH ⇌ CH₃COO⁻ + H⁺
Ionization constant (Ka) = [H⁺][CH₃COO⁻]/[CH₃COOH].
Similarly, base ionization constant (Kb).
3️⃣ Ostwald’s Dilution Law
Degree of dissociation (α) increases with dilution.
Ka = Cα² / (1 − α).
🟢 pH Concept (Sørensen, 1909)
pH = −log[H⁺]
Acidic solution: pH < 7.
Neutral: pH = 7.
Basic: pH > 7.
pOH = −log[OH⁻].
Relation: pH + pOH = 14 (at 298 K).
🔵 Ionization of Water
H₂O ⇌ H⁺ + OH⁻
Kw = [H⁺][OH⁻] = 1 × 10⁻¹⁴ (25°C).
📌 At neutrality: [H⁺] = [OH⁻] = 10⁻⁷ M.
🟡 Common Ion Effect
📌 Suppression of ionization of weak electrolyte by adding strong electrolyte containing common ion.
Example:
CH₃COOH ⇌ CH₃COO⁻ + H⁺
Addition of CH₃COONa (common ion CH₃COO⁻) suppresses ionization.
Used in buffer solutions and salt solubility control.
🔴 Buffer Solutions
Solutions which resist change in pH when small amounts of acid/base are added.
Types:
Acidic buffer: weak acid + salt (CH₃COOH + CH₃COONa).
Basic buffer: weak base + salt (NH₄OH + NH₄Cl).
📌 Henderson–Hasselbalch equation:
pH = pKa + log([salt]/[acid]).
🟢 Salt Hydrolysis
When salt reacts with water to form acidic/basic solutions.
Examples:
NaCl (strong acid + strong base) → neutral.
CH₃COONa (weak acid + strong base) → basic.
NH₄Cl (strong acid + weak base) → acidic.
🔵 Solubility Product (Ksp)
For sparingly soluble salts, solubility is small but equilibrium is established.
Example: AgCl ⇌ Ag⁺ + Cl⁻
Ksp = [Ag⁺][Cl⁻].
📌 If ionic product (Qsp) > Ksp → precipitation occurs.
🟡 Applications of Equilibrium Concepts
✔ Industrial synthesis: Haber (NH₃), Contact (H₂SO₄).
✔ Medicine: pH of blood (7.35–7.45) maintained by buffers.
✔ Environmental: Ocean pH, solubility of gases in water.
✔ Analytical: Precipitation titrations, solubility calculations.
✨ Conclusion
Equilibrium is a balance ⚖ between opposing forces. It explains why reactions don’t go to completion, how conditions influence yields, and why pH, buffers, solubility matter in chemistry and biology. It’s a foundation for understanding advanced topics like kinetics, electrochemistry, and biochemistry.
📌 Equilibrium – Key Takeaways
🔵 Physical Equilibria
Solid–liquid, liquid–vapor, solute–solution.
Dynamic, forward = backward rate.
🟢 Chemical Equilibria
Reversible reactions in closed systems.
Law of Mass Action → Kc expression.
Equilibrium constant: Kc, Kp; relation Kp = Kc(RT)^Δn.
🔴 Factors Affecting Equilibrium
Concentration, pressure, temperature.
Le Chatelier’s Principle: system shifts to oppose change.
🟡 Ionic Equilibrium
Strong vs weak electrolytes.
Ionization constants (Ka, Kb).
pH = −log[H⁺], pOH = −log[OH⁻], Kw = 10⁻¹⁴.
🌟 Special Effects
Common ion effect → ionization suppression.
Buffers resist pH changes (Henderson–Hasselbalch).
Salt hydrolysis: acidic, basic, neutral salts.
Solubility product (Ksp) → precipitation criteria.
📚 Significance
Industrial yields (NH₃, H₂SO₄).
Biological systems (blood pH).
Analytical chemistry (titrations, solubility).
📝 Quick Recap:
✔ Equilibrium is a dynamic balance in reversible reactions.
✔ Physical equilibria include melting, vaporization, dissolution.
✔ Chemical equilibria described by Kc, Kp; position depends on conditions.
✔ Le Chatelier’s Principle predicts shifts due to stress.
✔ Ionic equilibrium introduces pH, buffers, salt hydrolysis, Ksp.
✔ Common ion effect and buffer action crucial in labs and biology.
✔ Equilibrium is central to industry, medicine, and environment.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
🔵 Question 6.1:
A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
a) What is the initial effect of the change on vapour pressure?
b) How do rates of evaporation and condensation change initially?
c) What happens when equilibrium is restored finally and what will be the final vapour pressure?
🟢 Answer:
a) ➤ On increasing volume, vapour pressure decreases initially because the same number of vapour molecules now occupy a larger volume, so pressure falls.
b) ➤ Rate of evaporation remains constant (depends only on temperature) but rate of condensation decreases (fewer vapour molecules collide with liquid surface).
c) ➤ Since evaporation > condensation, more liquid evaporates. Vapour pressure increases again until new equilibrium is reached.
✔ Final vapour pressure = same as initial because it depends only on temperature, not volume.
🔵 Question 6.2:
What is Kc for the following equilibrium when the equilibrium concentration of each substance is: [SO₃] = 1.90 M, [SO₂] = 0.60 M, [O₂] = 0.82 M?
Reaction:
2SO₂(g) + O₂(g) ⇌ 2SO₃(g)
🟢 Answer:
➡ Expression: Kc = [SO₃]² / ([SO₂]² × [O₂])
➡ Substitute: Kc = (1.90)² / ((0.60)² × 0.82)
➡ Simplify: Kc = 3.61 / (0.36 × 0.82) = 3.61 / 0.2952
✔ Kc = 12.23
🔵 Question 6.3:
At a certain temperature and total pressure of 10⁵ Pa, iodine vapour contains 40% by volume of I atoms.
Reaction:
I₂(g) ⇌ 2I(g)
Calculate Kp for the equilibrium.
🟢 Answer:
Let total pressure = 10⁵ Pa
Fraction of I = 0.4 → I₂ = 0.6
Assume total moles = 1 →
I₂ = 0.6 mol, I = 0.4 mol
Total = 1 mol
Partial pressures:
p(I₂) = 0.6 × 10⁵ = 6 × 10⁴ Pa
p(I) = 0.4 × 10⁵ = 4 × 10⁴ Pa
Kp = (p(I))² / p(I₂)
= (4 × 10⁴)² / (6 × 10⁴)
= (16 × 10⁸) / (6 × 10⁴)
✔ Kp = 2.67 × 10⁴ Pa
🔵 Question 6.4:
Write the expression for the equilibrium constant, Kc, for each reaction:
(i) 2NOCl(g) ⇌ 2NO(g) + Cl₂(g)
(ii) 2Cu(NO₃)₂(s) ⇌ 2CuO(s) + 4NO₂(g) + O₂(g)
(iii) CH₃COOC₂H₅(aq) + H₂O(l) ⇌ CH₃COOH(aq) + C₂H₅OH(aq)
(iv) Fe³⁺(aq) + 3OH⁻(aq) ⇌ Fe(OH)₃(s)
(v) I₂(s) + 5F₂(g) ⇌ 2IF₅(g)
🟢 Answer:
(i) Kc = [NO]²[Cl₂] / [NOCl]²
(ii) Kc = [NO₂]⁴[O₂]
(Solids excluded)
(iii) Kc = [CH₃COOH][C₂H₅OH] / [CH₃COOC₂H₅]
(Water excluded as pure liquid)
(iv) Kc = 1 / ([Fe³⁺][OH⁻]³)
(v) Kc = [IF₅]² / [F₂]⁵
(Solid I₂ excluded)
🔵 Question 6.5:
Find the value of Kc from Kp for:
(i) 2NOCl(g) ⇌ 2NO(g) + Cl₂(g), Kp = 1.8 × 10⁻² at 500 K
(ii) CaCO₃(s) ⇌ CaO(s) + CO₂(g), Kp = 167 at 1073 K
🟢 Answer:
Formula: Kp = Kc(RT)^(Δn)
(i) Δn = (2 + 1) – 2 = 1
Kc = Kp / (RT)^1
= 1.8 × 10⁻² / (0.0821 × 500)
= 1.8 × 10⁻² / 41.05 = 4.38 × 10⁻⁴
(ii) Δn = 1 – 0 = 1
Kc = Kp / (RT)
= 167 / (0.0821 × 1073)
= 167 / 88.57 = 1.88
🔵 Question 6.6:
For equilibrium:
NO(g) + O₃(g) ⇌ NO₂(g) + O₂(g), Kc = 6.3 × 10¹⁴ at 1000 K
Find Kc for reverse reaction.
🟢 Answer:
For reverse reaction, Kc’ = 1 / Kc
✔ Kc’ = 1.59 × 10⁻¹⁵
🔵 Question 6.7:
Explain why pure liquids and solids can be ignored while writing equilibrium constant expression.
🟢 Answer:
✔ Concentration of pure solids and liquids is constant (density fixed).
✔ They are included in Kc value, but treated as 1.
Therefore, equilibrium expression includes only gases and solutes.
🔵 Question 6.8:
Reaction: 2N₂(g) + O₂(g) ⇌ 2N₂O(g)
Mixture: 0.482 mol N₂, 0.933 mol O₂ in 10 L, Kc = 2.0 × 10⁻³⁷
Determine equilibrium composition.
🟢 Answer:
➡ Initial conc: [N₂]₀ = 0.0482 M, [O₂]₀ = 0.0933 M, [N₂O]₀ = 0
Let x = formed conc of N₂O
At equilibrium:
[N₂] = 0.0482 – x
[O₂] = 0.0933 – 0.5x
[N₂O] = x
Kc = [N₂O]² / ([N₂]²[O₂])
As Kc is very small, x negligible.
So [N₂O] ≈ 0, [N₂] ≈ 0.0482 M, [O₂] ≈ 0.0933 M
✔ Reaction hardly proceeds.
🔵 Question 6.9:
2NO(g) + Br₂(g) ⇌ 2NOBr(g)
When 0.087 mol NO and 0.0437 mol Br₂ are mixed in 1 L, 0.0518 mol NOBr formed.
Find Kc.
🟢 Answer:
Initial: [NO] = 0.087, [Br₂] = 0.0437, [NOBr] = 0
Change: –0.0518 NOBr/2 = –0.0259 NO, –0.01295 Br₂
Equilibrium:
[NO] = 0.087 – 0.0518 = 0.0352
[Br₂] = 0.0437 – 0.0259 = 0.0178
[NOBr] = 0.0518
Kc = [NOBr]² / ([NO]²[Br₂])
= (0.0518)² / ((0.0352)² × 0.0178)
= 0.002684 / (0.001239 × 0.0178)
= 0.002684 / 2.2×10⁻⁵ = 122
🔵 Question 6.10:
At 450 K, Kp = 2.0 × 10¹⁰ for:
2SO₂(g) + O₂(g) ⇌ 2SO₃(g). Find Kc.
🟢 Answer:
Δn = (2) – (3) = –1
Kp = Kc(RT)^(Δn)
Kc = Kp × (RT)¹
= 2 × 10¹⁰ × (0.0821 × 450)
= 2 × 10¹⁰ × 36.945 = 7.39 × 10¹¹
🔵 Question 6.11:
HI(g) ⇌ H₂(g) + I₂(g)
At 0.2 atm total pressure, p(HI)=0.04 atm
Find Kp.
🟢 Answer:
Let dissociation = x
p(HI) = 0.2 – 2x = 0.04 → x = 0.08
p(H₂) = x = 0.08, p(I₂) = x = 0.08
Kp = (0.08 × 0.08) / (0.04)² = 0.0064 / 0.0016 = 4
🔵 Question 6.12:
N₂ + 3H₂ ⇌ 2NH₃
1.57 mol N₂, 1.92 mol H₂, 8.13 mol NH₃ in 20 L at 500 K
Kc = 1.7 × 10²
Check equilibrium.
🟢 Answer:
Concentrations:
[N₂] = 1.57/20 = 0.0785
[H₂] = 1.92/20 = 0.096
[NH₃] = 8.13/20 = 0.4065
Q = [NH₃]² / ([N₂][H₂]³)
= (0.4065)² / (0.0785 × 0.096³)
= 0.165 / (0.0785 × 0.0008847)
= 0.165 / 6.94×10⁻⁵ = 2377
Q > Kc → reaction shifts left.
🔵 Question 6.13:
Given Kc = [NH₄⁺][OH⁻] / ([NO][H₃O⁺])
Write balanced equation.
🟢 Answer:
NH₄NO ⇌ NH₄⁺ + NO + H₃O⁺ + OH⁻
But to match form, reaction is:
NH₄NO + H₂O ⇌ NH₄⁺ + NO + H₃O⁺ + OH⁻
🔵 Question 6.14:
1 mol H₂O + 1 mol CO in 10 L at 725 K
40% H₂O reacts → 0.4 mol reacts
Reaction: H₂O + CO ⇌ H₂ + CO₂
Equilibrium moles:
H₂O = 0.6, CO = 0.6, H₂ = 0.4, CO₂ = 0.4
Concentrations:
All /10 → [H₂O] = 0.06, [CO] = 0.06, [H₂] = 0.04, [CO₂] = 0.04
Kc = [H₂][CO₂]/([H₂O][CO])
= (0.04×0.04)/(0.06×0.06)
= 0.0016/0.0036 = 0.444
🔵 Question 6.15:
At 700 K, for HI ⇌ ½H₂ + ½I₂, K = 54.8
If [HI]=0.5 M, find [H₂] and [I₂].
🟢 Answer:
HI ⇌ H₂ + I₂
Let dissociation = x
Kc = [H₂][I₂]/[HI]² = x²/(0.5–2x)²
Approximate if x small: x = √(Kc)×[HI] = √(54.8)×0.5 ≈ 7.4×0.5 = 3.7
Not possible; hence solve exact:
Let [HI]=0.5–2x
Kc=54.8=(x²)/(0.5–2x)²
√54.8 = x/(0.5–2x)
7.4(0.5–2x)=x → 3.7–14.8x=x → 15.8x=3.7 → x=0.234
So [H₂]=[I₂]=0.234 M, [HI]=0.5–0.468=0.032 M
✔ [H₂]=0.234 M, [I₂]=0.234 M, [HI]=0.032 M
🔵 Question 6.16
What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M?
Reaction: 2 ICl (g) ⇌ I₂ (g) + Cl₂ (g); Kc = 0.14
🟢 Answer
Let dissociation = 2x M.
Initial: [ICl] = 0.78, [I₂] = 0, [Cl₂] = 0
Change: –2x, +x, +x
Equilibrium: [ICl] = 0.78 – 2x, [I₂] = x, [Cl₂] = x
Kc = ([I₂][Cl₂]) / [ICl]² = x² / (0.78 – 2x)² = 0.14
√0.14 = x / (0.78 – 2x) → 0.374(0.78 – 2x) = x
0.291 – 0.748x = x → 1.748x = 0.291 → x = 0.166 M
✅ [I₂] = [Cl₂] = 0.166 M; [ICl] = 0.448 M
🔵 Question 6.17
For C₂H₆ (g) ⇌ C₂H₄ (g) + H₂ (g), Kp = 0.04 atm at 899 K.
If the initial pressure of C₂H₆ is 4 atm, find its equilibrium pressure.
🟢 Answer
Let dissociation = x atm.
P(C₂H₆) = 4 – x; P(C₂H₄) = x; P(H₂) = x
Kp = x² / (4 – x) = 0.04 ⇒ x ≈ 0.4 atm
✅ P(C₂H₆) = 3.6 atm, P(C₂H₄) = 0.4 atm, P(H₂) = 0.4 atm
🔵 Question 6.18
CH₃COOH (l) + C₂H₅OH (l) ⇌ CH₃COOC₂H₅ (l) + H₂O (l)
(i) Write Qc.
(ii) If 0.171 mol ester is formed from 1 mol each of reactants in 1 L at 293 K, find Kc.
(iii) If later ester = 0.214 mol, has equilibrium been reached?
🟢 Answer
(i) Qc = [ester][H₂O]/([acid][alcohol])
(ii) Equilibrium: acid = alcohol = 0.829 M; products = 0.171 M
Kc = (0.171²)/(0.829²) = 0.042
(iii) For 0.214 mol, Qc = 0.074 > Kc ⇒ shifts backward ⇒ not at equilibrium.
🔵 Question 6.19
PCl₅ (g) ⇌ PCl₃ (g) + Cl₂ (g), Kc = 8.3 × 10⁻³ at 473 K.
If 1 mol PCl₅ in 1 L partially dissociates, find equilibrium concentrations.
🟢 Answer
Let dissociation = x mol.
[PCl₅] = 1 – x, [PCl₃] = x, [Cl₂] = x
Kc = x² / (1 – x) = 8.3×10⁻³ ⇒ x ≈ 0.091 M
✅ [PCl₅] = 0.909 M, [PCl₃] = 0.091 M, [Cl₂] = 0.091 M
🔵 Question 6.20
FeO (s) + CO (g) ⇌ Fe (s) + CO₂ (g), Kp = 0.265 at 1050 K.
Initial: PCO = 1.4 atm, PCO₂ = 0.80 atm. Find equilibrium pressures.
🟢 Answer
Let change = x.
1.4 – x, 0.80 + x satisfy 0.265 = (0.80 + x)/(1.4 – x)
x = –0.339 ⇒ backward shift
✅ PCO = 1.739 atm, PCO₂ = 0.461 atm
🔵 Question 6.21
N₂ + 3H₂ ⇌ 2NH₃, Kc = 0.061.
Given [N₂] = 3 M, [H₂] = 2 M, [NH₃] = 0.5 M. Is system at equilibrium?
🟢 Answer
Qc = 0.25 / (3 × 8) = 0.0104 < Kc ⇒ reaction moves forward.
🔵 Question 6.22
2BrCl ⇌ Br₂ + Cl₂, Kc = 3.2×10⁻³; initial [BrCl] = 3.3×10⁻³ M.
Find equilibrium concentrations.
🟢 Answer
x = 1.87×10⁻⁴ M
✅ [Br₂] = [Cl₂] = 1.87×10⁻⁴ M; [BrCl] = 2.93×10⁻³ M
🔵 Question 6.23
C (s) + CO₂ (g) ⇌ 2CO (g); mixture at 1 atm has 90.55 % CO by mass. Find Kp.
🟢 Answer
Mole fractions: X_CO = 0.938, X_CO₂ = 0.062
Kp = (0.938)² / 0.062 = 14.2 atm
🔵 Question 6.24
NO + ½O₂ ⇌ NO₂; ΔG°(NO₂)=52, ΔG°(NO)=87 kJ mol⁻¹.
Find ΔG° and K.
🟢 Answer
ΔG° = 52 – 87 = –35 kJ
K = e^(35000 / (8.314×298)) ≈ 1.3×10⁶
✔ ΔG° = –35 kJ, K = 1.3×10⁶
🔵 Question 6.25
How does decreasing pressure affect equilibrium when gas moles differ?
🟢 Answer
Shift to side with more gas moles.
Example: N₂O₄ ⇌ 2NO₂ → shifts right.
🔵 Question 6.26
Which equilibria shift on increasing pressure?
🟢 Answer
Shift to side with fewer moles.
→ Reactions (iv) forward; (ii, iii, vi) backward; (i, v) no effect.
🔵 Question 6.27
H₂ + Br₂ ⇌ 2HBr, Kp = 1.6×10⁵, initial P(HBr)=10 bar.
Find equilibrium composition.
🟢 Answer
Let dissociation = 2x.
(10 – 2x)²/x² = 1.6×10⁵ ⇒ x ≈ 0.025 bar
✅ P(HBr) = 9.95 bar; P(H₂) = P(Br₂) = 0.025 bar
🔵 Question 6.28
CH₄ + H₂O ⇌ CO + 3H₂ (endothermic)
🟢 Answer
Kp = (P_CO × P_H₂³)/(P_CH₄ × P_H₂O)
↑P → left; ↑T → right; catalyst → no shift.
🔵 Question 6.29
2H₂ + CO ⇌ CH₃OH
🟢 Answer
Add H₂ → right; add CH₃OH → left; remove CO → left; remove CH₃OH → right.
🔵 Question 6.30
PCl₅ ⇌ PCl₃ + Cl₂, Kp = 8.3×10⁻³, ΔH° = +124 kJ.
🟢 Answer
(a) Kc = [PCl₃][Cl₂]/[PCl₅]
(b) Reverse K = 120.5
(c) Add PCl₅ → forward; ↑P → left; ↑T → right (K increases)
🔵 Question 6.31
Explain the following terms:
(a) Conjugate acid-base pair
(b) Amphoteric species
(c) Lewis acids and bases
🟢 Answer:
(a) Conjugate acid-base pair:
When an acid donates a proton (H⁺), the species formed is its conjugate base; similarly, when a base accepts a proton, the species formed is its conjugate acid.
➡ Example: HCl + H₂O ⇌ H₃O⁺ + Cl⁻
Here, HCl (acid) → Cl⁻ (conjugate base), H₂O (base) → H₃O⁺ (conjugate acid).
Pair: (HCl / Cl⁻), (H₂O / H₃O⁺)
(b) Amphoteric species:
A species that can act as both acid and base.
➡ Example: HCO₃⁻ acts as acid (donates H⁺) and base (accepts H⁺).
(c) Lewis acids and bases:
Lewis acid: Electron pair acceptor (e.g., BF₃, AlCl₃).
Lewis base: Electron pair donor (e.g., NH₃, OH⁻).
➡ Example: BF₃ + NH₃ → F₃B←NH₃
🔵 Question 6.32
The ionization constant of HF is 6.8×10⁻⁴. Calculate the degree of dissociation of HF in 0.1 M solution and pH.
🟢 Answer:
Let HF ⇌ H⁺ + F⁻
Initial = 0.1, 0, 0
Change = –α×0.1, +α×0.1, +α×0.1
Ka = (Cα²)/(1–α) ≈ Cα² (since α small)
6.8×10⁻⁴ = 0.1α²
α² = 6.8×10⁻³ → α = 0.082
✔ Degree of dissociation = 8.2%
[H⁺] = Cα = 0.1 × 0.082 = 8.2×10⁻³ M
pH = –log(8.2×10⁻³) = 2.09
🔵 Question 6.33
Calculate pH of 1×10⁻⁸ M HCl solution.
🟢 Answer:
H⁺ comes from both acid and water:
Let total [H⁺] = x
x = [H⁺ from HCl] + [H⁺ from H₂O] = 1×10⁻⁸ + (Kw/x)
Kw = 1×10⁻¹⁴
⇒ x² – 1×10⁻⁸x – 1×10⁻¹⁴ = 0
Solve: x = (1×10⁻⁸ + √(1×10⁻¹⁶ + 4×10⁻¹⁴)) / 2
x = (1×10⁻⁸ + 2×10⁻⁷)/2 ≈ 1.05×10⁻⁷ M
pH = –log(1.05×10⁻⁷) = 6.98
✔ Slightly < 7 (acidic)
🔵 Question 6.34
The ionization constant of phenol is 1×10⁻¹⁰. What is the concentration of phenolate ion in 0.05 M phenol solution? Also find pH.
🟢 Answer:
C₆H₅OH ⇌ C₆H₅O⁻ + H⁺
Ka = 1×10⁻¹⁰ = (x²)/(0.05) ⇒ x² = 5×10⁻¹² ⇒ x = 2.24×10⁻⁶
✔ [C₆H₅O⁻] = 2.24×10⁻⁶ M
pH = –log(2.24×10⁻⁶) = 5.65
🔵 Question 6.35
What is the pH of 0.002 M solution of NaOH?
🟢 Answer:
NaOH is strong base → [OH⁻] = 0.002 M
pOH = –log(0.002) = 2.70
pH = 14 – 2.70 = 11.30
🔵 Question 6.36
Calculate pH of 0.001 M Ba(OH)₂ solution.
🟢 Answer:
Each Ba(OH)₂ gives 2 OH⁻
[OH⁻] = 2 × 0.001 = 0.002 M
pOH = –log(0.002) = 2.70
pH = 14 – 2.70 = 11.30
🔵 Question 6.37
Calculate pH of 0.1 M solution of an acid with Ka = 1.0×10⁻⁴.
🟢 Answer:
HA ⇌ H⁺ + A⁻
Ka = Cα² = 1×10⁻⁴ = 0.1α² → α² = 1×10⁻³ → α = 0.0316
[H⁺] = Cα = 0.1 × 0.0316 = 3.16×10⁻³ M
pH = –log(3.16×10⁻³) = 2.50
🔵 Question 6.38
Calculate pH of 0.01 M H₂SO₄ solution (considering full dissociation of first H⁺ and partial for second).
Ka₂ = 1.2×10⁻²
🟢 Answer:
After first dissociation: [H⁺] = 0.01, [HSO₄⁻] = 0.01
HSO₄⁻ ⇌ H⁺ + SO₄²⁻
Let additional H⁺ = x
Ka₂ = (0.01 + x)(x)/(0.01 – x) ≈ (0.01)(x)/0.01 = x = 1.2×10⁻²
Total [H⁺] = 0.01 + 0.012 = 0.022 M
pH = –log(0.022) = 1.66
🔵 Question 6.39
What is the pH of 10⁻⁸ M NaOH solution?
🟢 Answer:
Let [OH⁻] = x = 10⁻⁸
Total [OH⁻] = 10⁻⁸ + (Kw/x) = 10⁻⁸ + 10⁻⁶ = 1.01×10⁻⁶
pOH = –log(1.01×10⁻⁶) = 5.996
pH = 14 – 5.996 = 8.00
✔ Slightly basic.
🔵 Question 6.40
Calculate pH of 0.2 M solution of HCN (Ka = 4.8×10⁻¹⁰).
🟢 Answer:
Ka = Cα² ⇒ α² = 4.8×10⁻¹⁰ / 0.2 = 2.4×10⁻⁹
α = 4.9×10⁻⁵
[H⁺] = 0.2 × 4.9×10⁻⁵ = 9.8×10⁻⁶
pH = –log(9.8×10⁻⁶) = 5.01
🔵 Question 6.41
The pH of a weak acid solution is 3.0. Calculate its ionization constant if concentration is 0.1 M.
🟢 Answer:
pH = 3 → [H⁺] = 1×10⁻³
Ka = [H⁺]² / (C – [H⁺]) = (1×10⁻³)² / (0.1 – 0.001) = 1×10⁻⁶ / 0.099 ≈ 1.01×10⁻⁵
🔵 Question 6.42
Find pH of 0.05 M CH₃COOH (Ka = 1.8×10⁻⁵).
🟢 Answer:
Ka = Cα² ⇒ α² = 1.8×10⁻⁵ / 0.05 = 3.6×10⁻⁴ ⇒ α = 0.019
[H⁺] = 0.05 × 0.019 = 9.5×10⁻⁴
pH = –log(9.5×10⁻⁴) = 3.02
🔵 Question 6.43
Find pH of a solution obtained by mixing 50 mL of 0.1 M HCl with 50 mL of 0.1 M NaOH.
🟢 Answer:
Moles HCl = 0.005, Moles NaOH = 0.005 → neutralize completely
Solution = 100 mL, [H⁺] = 0
Neutral → pH = 7.00
🔵 Question 6.44
Find pH of a solution obtained by mixing 25 mL of 0.1 M HCl with 50 mL of 0.1 M NaOH.
🟢 Answer:
Moles HCl = 0.0025, Moles NaOH = 0.005 → Excess OH⁻ = 0.0025 mol
Total volume = 75 mL = 0.075 L
[OH⁻] = 0.0025 / 0.075 = 0.0333 M
pOH = –log(0.0333) = 1.48
pH = 14 – 1.48 = 12.52
🔵 Question 6.45
Calculate pH of a solution obtained by mixing 40 mL of 0.1 M NaOH with 10 mL of 0.1 M HCl.
🟢 Answer:
Moles NaOH = 0.004, HCl = 0.001 → Excess OH⁻ = 0.003 mol
Total volume = 50 mL = 0.05 L
[OH⁻] = 0.003 / 0.05 = 0.06 M
pOH = –log(0.06) = 1.22
pH = 14 – 1.22 = 12.78
🔵 Question 6.46
Calculate the pH of a buffer solution containing 0.1 M NH₃ and 0.1 M NH₄Cl. (Kb for NH₃ = 1.77 × 10⁻⁵)
🟢 Answer:
We use Henderson–Hasselbalch equation (for basic buffer):
pOH = pKb + log([salt]/[base])
➡ pKb = –log(1.77 × 10⁻⁵) = 4.75
➡ Ratio [salt]/[base] = 0.1 / 0.1 = 1
So pOH = 4.75 + log(1) = 4.75
pH = 14 – 4.75 = 9.25
✔ pH = 9.25
🔵 Question 6.47
Calculate the pH of a buffer solution containing 0.05 M acetic acid and 0.1 M sodium acetate. (Ka = 1.8 × 10⁻⁵)
🟢 Answer:
Use Henderson–Hasselbalch (acidic buffer):
pH = pKa + log([salt]/[acid])
pKa = –log(1.8 × 10⁻⁵) = 4.74
Ratio = 0.1 / 0.05 = 2
pH = 4.74 + log(2) = 4.74 + 0.30 = 5.04
✔ pH = 5.04
🔵 Question 6.48
How much NaOH must be added to 0.5 L of 0.1 M acetic acid to make pH = 4.74? (Ka = 1.8 × 10⁻⁵)
🟢 Answer:
For pH = pKa → ratio [salt]/[acid] = 1
Initial moles acid = 0.5 × 0.1 = 0.05 mol
We need [salt] = [acid] ⇒ convert half acid into salt
Moles NaOH required = 0.025 mol
Mass = 0.025 × 40 = 1.0 g
✔ Add 1.0 g NaOH
🔵 Question 6.49
What is the pH of a solution obtained by mixing 100 mL of 0.1 M CH₃COOH and 100 mL of 0.1 M NaOH? (Ka = 1.8 × 10⁻⁵)
🟢 Answer:
Moles CH₃COOH = 0.01, NaOH = 0.01 → neutralize completely
→ forms 0.01 mol CH₃COONa in 0.2 L → [salt] = 0.05 M
Buffer with no free acid/base, pH ≈ pKa + log([salt]/[acid])
[acid] ≈ 0 → treat as weak base hydrolysis:
Kb = Kw / Ka = 10⁻¹⁴ / 1.8×10⁻⁵ = 5.56×10⁻¹⁰
[OH⁻] = √(Kb × C) = √(5.56×10⁻¹⁰ × 0.05) = 5.3×10⁻⁶
pOH = 5.28 → pH = 8.72
✔ pH = 8.72
🔵 Question 6.50
Find pH of a mixture of 50 mL 0.1 M NH₄OH and 25 mL 0.1 M HCl. (Kb = 1.77 × 10⁻⁵)
🟢 Answer:
Moles NH₄OH = 0.005, HCl = 0.0025
Excess base = 0.0025 mol; salt formed = 0.0025 mol
Total volume = 75 mL = 0.075 L
[base] = 0.0025 / 0.075 = 0.0333 M
[salt] = 0.0333 M
pOH = pKb + log([salt]/[base]) = 4.75 + log(1) = 4.75
pH = 14 – 4.75 = 9.25
✔ pH = 9.25
🔵 Question 6.51
What is the pH of a solution obtained by mixing 50 mL of 0.1 M NaOH and 25 mL of 0.1 M HCl?
🟢 Answer:
Moles NaOH = 0.005, HCl = 0.0025
Excess OH⁻ = 0.0025 mol
Total volume = 75 mL = 0.075 L
[OH⁻] = 0.0025 / 0.075 = 0.0333 M
pOH = –log(0.0333) = 1.48
pH = 14 – 1.48 = 12.52
✔ pH = 12.52
🔵 Question 6.52
Calculate the pH of a solution prepared by mixing 25 mL of 0.1 M NaOH with 50 mL of 0.1 M HCl.
🟢 Answer:
Moles NaOH = 0.0025, HCl = 0.005 → excess acid = 0.0025 mol
Total volume = 75 mL = 0.075 L
[H⁺] = 0.0025 / 0.075 = 0.0333 M
pH = –log(0.0333) = 1.48
✔ pH = 1.48
🔵 Question 6.53
What is the pH of a buffer prepared by mixing 250 mL of 0.1 M NH₄OH and 250 mL of 0.1 M NH₄Cl? (Kb = 1.77×10⁻⁵)
🟢 Answer:
Equal moles of base and salt → [salt]/[base] = 1
pOH = pKb = 4.75
pH = 14 – 4.75 = 9.25
✔ pH = 9.25
🔵 Question 6.54
Calculate pH of a solution obtained by mixing 40 mL 0.1 M HCl and 10 mL 0.1 M NaOH.
🟢 Answer:
Moles HCl = 0.004, NaOH = 0.001 → excess acid = 0.003
Total volume = 50 mL = 0.05 L
[H⁺] = 0.003 / 0.05 = 0.06 M
pH = –log(0.06) = 1.22
✔ pH = 1.22
🔵 Question 6.55
Calculate pH of a solution obtained by mixing 10 mL 0.1 M HCl and 40 mL 0.1 M NaOH.
🟢 Answer:
Moles HCl = 0.001, NaOH = 0.004 → excess OH⁻ = 0.003
Total volume = 50 mL = 0.05 L
[OH⁻] = 0.003 / 0.05 = 0.06 M
pOH = –log(0.06) = 1.22
pH = 14 – 1.22 = 12.78
✔ pH = 12.78
🔵 Question 6.56
Calculate the pH of a 0.05 M NH₄Cl solution. (Kb for NH₃ = 1.77×10⁻⁵)
🟢 Answer:
NH₄Cl → weak acid (hydrolysis of NH₄⁺):
Ka = Kw / Kb = 10⁻¹⁴ / 1.77×10⁻⁵ = 5.65×10⁻¹⁰
[H⁺] = √(Ka × C) = √(5.65×10⁻¹⁰ × 0.05) = 5.31×10⁻⁶
pH = –log(5.31×10⁻⁶) = 5.28
✔ pH = 5.28
🔵 Question 6.57
Calculate the pH of 0.1 M Na₂CO₃ solution. (Kb₁ for CO₃²⁻ = 2.1×10⁻⁴)
🟢 Answer:
CO₃²⁻ + H₂O ⇌ HCO₃⁻ + OH⁻
[OH⁻] = √(Kb₁ × C) = √(2.1×10⁻⁴ × 0.1) = 4.58×10⁻³
pOH = –log(4.58×10⁻³) = 2.34
pH = 14 – 2.34 = 11.66
✔ pH = 11.66
🔵 Question 6.58
Calculate the pH of 0.1 M NaHCO₃ solution. (Ka₁ = 4.3×10⁻⁷, Ka₂ = 4.8×10⁻¹¹)
🟢 Answer:
HCO₃⁻ is amphiprotic → pH = ½(pKa₁ + pKa₂)
pKa₁ = 6.37, pKa₂ = 10.32
pH = ½(6.37 + 10.32) = 8.35
✔ pH = 8.35
🔵 Question 6.59
What is the pH of 0.1 M CH₃COONa solution? (Ka = 1.8×10⁻⁵)
🟢 Answer:
CH₃COO⁻ hydrolysis: Kb = Kw / Ka = 10⁻¹⁴ / 1.8×10⁻⁵ = 5.56×10⁻¹⁰
[OH⁻] = √(Kb × C) = √(5.56×10⁻¹⁰ × 0.1) = 7.45×10⁻⁶
pOH = 5.13 → pH = 14 – 5.13 = 8.87
✔ pH = 8.87
🔵 Question 6.60
Calculate the pH of 0.05 M NH₄NO₃ solution. (Kb for NH₃ = 1.77×10⁻⁵)
🟢 Answer:
NH₄⁺ hydrolysis: Ka = Kw / Kb = 10⁻¹⁴ / 1.77×10⁻⁵ = 5.65×10⁻¹⁰
[H⁺] = √(Ka × C) = √(5.65×10⁻¹⁰ × 0.05) = 5.31×10⁻⁶
pH = –log(5.31×10⁻⁶) = 5.28
✔ pH = 5.28
🔵 Question 6.61
Calculate the pH of 0.1 M NH₄Cl and 0.05 M NaOH mixture. (Kb for NH₃ = 1.77 × 10⁻⁵)
🟢 Answer:
This forms a basic buffer (NH₄⁺/NH₃).
Use pOH = pKb + log([salt]/[base])
pKb = –log(1.77 × 10⁻⁵) = 4.75
Ratio = 0.1 / 0.05 = 2
pOH = 4.75 + log(2) = 4.75 + 0.30 = 5.05
pH = 14 – 5.05 = 8.95
✔ pH = 8.95
🔵 Question 6.62
Find pH of a buffer containing 0.1 M CH₃COOH and 0.1 M CH₃COONa when 0.01 mol HCl is added to 1 L. (Ka = 1.8 × 10⁻⁵)
🟢 Answer:
Initial moles acid = 0.1; salt = 0.1
HCl converts salt → acid:
New moles acid = 0.11; salt = 0.09
pH = pKa + log([salt]/[acid])
pKa = 4.74
pH = 4.74 + log(0.09/0.11) = 4.74 + log(0.818) = 4.74 – 0.087 = 4.65
✔ pH = 4.65
🔵 Question 6.63
Find pH of same buffer when 0.01 mol NaOH is added instead.
🟢 Answer:
NaOH reacts with acid → salt:
New acid = 0.09, salt = 0.11
pH = 4.74 + log(0.11/0.09) = 4.74 + 0.087 = 4.83
✔ pH = 4.83
🔵 Question 6.64
Calculate solubility of AgCl (Ksp = 1.6 × 10⁻¹⁰).
🟢 Answer:
AgCl ⇌ Ag⁺ + Cl⁻
Let solubility = s
Ksp = s²
s = √(1.6 × 10⁻¹⁰) = 1.26 × 10⁻⁵ M
✔ Solubility = 1.26 × 10⁻⁵ mol L⁻¹
🔵 Question 6.65
Calculate solubility of PbCl₂ (Ksp = 1.7 × 10⁻⁵).
🟢 Answer:
PbCl₂ ⇌ Pb²⁺ + 2 Cl⁻
Let solubility = s
Ksp = s × (2s)² = 4s³
s³ = 1.7 × 10⁻⁵ / 4 = 4.25 × 10⁻⁶
s = (4.25 × 10⁻⁶)^(1/3) = 1.62 × 10⁻² M
✔ Solubility = 1.62 × 10⁻² mol L⁻¹
🔵 Question 6.66
Calculate solubility of CaF₂ in water (Ksp = 3.2 × 10⁻¹¹).
🟢 Answer:
CaF₂ ⇌ Ca²⁺ + 2 F⁻
Ksp = s × (2s)² = 4s³
s³ = 3.2 × 10⁻¹¹ / 4 = 8 × 10⁻¹²
s = (8 × 10⁻¹²)^(1/3) = 2.0 × 10⁻⁴ M
✔ Solubility = 2.0 × 10⁻⁴ mol L⁻¹
🔵 Question 6.67
Calculate solubility of Ag₂CrO₄ (Ksp = 1.1 × 10⁻¹²).
🟢 Answer:
Ag₂CrO₄ ⇌ 2 Ag⁺ + CrO₄²⁻
Ksp = (2s)² × s = 4s³
s³ = 1.1 × 10⁻¹² / 4 = 2.75 × 10⁻¹³
s = (2.75 × 10⁻¹³)^(1/3) = 6.5 × 10⁻⁵ M
✔ Solubility = 6.5 × 10⁻⁵ mol L⁻¹
🔵 Question 6.68
Calculate solubility of BaSO₄ (Ksp = 1.1 × 10⁻¹⁰) in 0.01 M Na₂SO₄.
🟢 Answer:
BaSO₄ ⇌ Ba²⁺ + SO₄²⁻
Ksp = s × (0.01 + s) ≈ s × 0.01
s = 1.1 × 10⁻¹⁰ / 0.01 = 1.1 × 10⁻⁸ M
✔ Solubility = 1.1 × 10⁻⁸ mol L⁻¹
🔵 Question 6.69
Calculate solubility of AgCl in 0.1 M NaCl. (Ksp = 1.6 × 10⁻¹⁰)
🟢 Answer:
Ksp = [Ag⁺][Cl⁻] ≈ s × 0.1
s = 1.6 × 10⁻¹⁰ / 0.1 = 1.6 × 10⁻⁹ M
✔ Solubility = 1.6 × 10⁻⁹ mol L⁻¹
🔵 Question 6.70
If Ksp = 1.0 × 10⁻²⁵ for Ag₂S, find solubility in pure water.
🟢 Answer:
Ag₂S ⇌ 2 Ag⁺ + S²⁻
Ksp = 4s³
s³ = 1 × 10⁻²⁵ / 4 = 2.5 × 10⁻²⁶
s = (2.5 × 10⁻²⁶)^(1/3) = 2.9 × 10⁻⁹ M
✔ Solubility = 2.9 × 10⁻⁹ mol L⁻¹
🔵 Question 6.71
What is the pH at which Fe(OH)₃ (Ksp = 4 × 10⁻³⁸) starts precipitating from 0.001 M Fe³⁺?
🟢 Answer:
Ksp = [Fe³⁺][OH⁻]³
[OH⁻]³ = 4 × 10⁻³⁸ / 10⁻³ = 4 × 10⁻³⁵
[OH⁻] = (4 × 10⁻³⁵)^(1/3) = 3.4 × 10⁻¹²
pOH = 11.47 → pH = 2.53
✔ pH = 2.53
🔵 Question 6.72
At what pH will Mg(OH)₂ (Ksp = 8.9 × 10⁻¹²) start to precipitate from 0.01 M Mg²⁺?
🟢 Answer:
Ksp = [Mg²⁺][OH⁻]²
[OH⁻]² = 8.9 × 10⁻¹² / 0.01 = 8.9 × 10⁻¹⁰
[OH⁻] = 2.98 × 10⁻⁵ → pOH = 4.53 → pH = 9.47
✔ pH = 9.47
🔵 Question 6.73
Will AgCl (Ksp = 1.6 × 10⁻¹⁰) precipitate if 10⁻⁴ M AgNO₃ and 10⁻⁴ M NaCl are mixed?
🟢 Answer:
Ionic product, Q = [Ag⁺][Cl⁻] = (10⁻⁴)(10⁻⁴) = 10⁻⁸
Q > Ksp (10⁻⁸ > 1.6 × 10⁻¹⁰) → precipitation occurs
✔ AgCl will precipitate.
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
(CBSE MODEL QUESTIONS PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
✳ Section A (Q1–Q16) – MCQs (1 mark each, 16 × 1 = 16 marks)
Options:
Both Assertion (A) and Reason (R) are true, and R is the correct explanation of A
Both A and R are true, but R is not the correct explanation of A
A is true, but R is false
A is false, but R is true
Question 1. Equilibrium in a chemical reaction is established when:
Forward and backward reactions stop
Forward and backward reactions have equal rates
Concentration of reactants becomes zero
Products stop forming
Answer: 2
Question 2. Which of the following is a dynamic equilibrium?
Evaporation of water in a closed vessel
Evaporation in an open vessel
Decomposition of H₂O₂ in open air
Rusting of iron
Answer: 1
Question 3. The equilibrium constant is expressed as:
K_p/K_c
Products/Reactants
Reactants/Products
None of these
Answer: 2
Question 4. For the reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), the equilibrium constant expression K_c is:
[N₂][H₂]³/[NH₃]²
[NH₃]²/[N₂][H₂]³
[NH₃]/[N₂][H₂]
[NH₃]³/[N₂][H₂]²
Answer: 2
Question 5. The value of K_c for a reaction is 10⁵. This means:
Products are favored
Reactants are favored
Both equally favored
Equilibrium does not exist
Answer: 1
Question 6. If Q_c > K_c, the reaction will:
Proceed forward
Proceed backward
Remain at equilibrium
Stop
Answer: 2
Question 7. pH of neutral water at 25 °C is:
6
7
8
14
Answer: 2
Question 8. Kw at 25 °C is equal to:
10⁻¹⁴
10⁻⁷
10⁻¹²
10⁻¹⁰
Answer: 1
Question 9. Which acid is diprotic?
HCl
H₂SO₄
HNO₃
CH₃COOH
Answer: 2
Question 10. Common-ion effect is used in:
Neutralization
Buffer solutions
Hydrolysis
Titrations only
Answer: 2
Question 11. Which salt solution is basic?
NaCl
NH₄Cl
CH₃COONa
KHSO₄
Answer: 3
Question 12. The unit of K_c for reaction A + B ⇌ AB is:
mol L⁻¹
L mol⁻¹
Dimensionless
mol² L⁻²
Answer: 2
Question 13. Le Chatelier’s principle predicts:
Direction of reaction shift
Equilibrium constant value
Activation energy
Bond energy
Answer: 1
Question 14. (Assertion–Reason)
Assertion (A): For exothermic reactions, increasing temperature decreases K.
Reason (R): Equilibrium shifts in direction that absorbs heat.
Answer: 1
Question 15. (Assertion–Reason)
Assertion (A): Pure solids and liquids are not included in K expressions.
Reason (R): Their concentrations remain constant.
Answer: 1
Question 16. The pH of 0.01 M HCl solution is:
1
2
3
4
Answer: 2
⚡ Section B (Q17–Q21) – Very Short Answer (2 marks each, 5 × 2 = 10 marks)
Q17. Define equilibrium constant.
🟦 The ratio of product concentration to reactant concentration at equilibrium, each raised to power of stoichiometric coefficients.
🟩 Represents position of equilibrium.
Q18. Write expression for K_p in terms of K_c.
🟦 K_p = K_c(RT)^Δn
🟩 Δn = moles of gaseous products – moles of gaseous reactants.
Q19. What is the significance of Q_c?
🟦 Q_c = [products]/[reactants] at any instant.
🟩 Comparison of Q_c and K_c tells direction of reaction shift.
Q20. Calculate pOH of a solution with [OH⁻] = 1 × 10⁻⁵ M.
➤ Formula: pOH = –log[OH⁻]
➤ Substitution: pOH = –log(1 × 10⁻⁵)
✅ Final Answer: pOH = 5
Q21. State common-ion effect with an example.
🟦 Suppression of ionization of weak electrolyte by adding strong electrolyte containing a common ion.
🟩 Example: CH₃COOH ionization suppressed by CH₃COONa.
🧪 Section C (Q22–Q28) – Short Answer (3 marks each, 7 × 3 = 21 marks)
Q22. Differentiate between homogeneous and heterogeneous equilibrium with examples.
🟦 Homogeneous: all species in same phase (N₂ + 3H₂ ⇌ 2NH₃).
🟨 Heterogeneous: species in different phases (CaCO₃(s) ⇌ CaO(s) + CO₂(g)).
🟩 Criterion: phases present.
Q23. Derive relationship between K_p and K_c.
➤ General reaction: aA + bB ⇌ cC + dD.
➤ K_p = (pC^c pD^d)/(pA^a pB^b).
➤ Using p = cRT: K_p = K_c(RT)^Δn.
✅ Relation: K_p = K_c(RT)^Δn.
Q24. Explain effect of temperature on equilibrium constant for exothermic and endothermic reactions.
🟦 Exothermic: Increase in T → K decreases, reaction shifts backward.
🟨 Endothermic: Increase in T → K increases, reaction shifts forward.
🟩 Explained by Le Chatelier’s principle.
Q25. Write differences between strong and weak electrolytes with examples.
🟦 Strong: completely ionize in solution (HCl, NaOH).
🟨 Weak: partially ionize (CH₃COOH, NH₃).
🟩 Conductivity higher for strong electrolytes.
Q26. Calculate pH of 0.001 M HNO₃ solution.
➤ Formula: pH = –log[H⁺].
➤ Substitution: pH = –log(0.001).
✅ Final Answer: pH = 3.
Q27. Explain buffer action of CH₃COOH/CH₃COONa.
🟦 Addition of H⁺ → reacts with CH₃COO⁻ to form CH₃COOH.
🟨 Addition of OH⁻ → reacts with CH₃COOH to form CH₃COO⁻.
🟩 Maintains nearly constant pH.
Q28. Write expression for hydrolysis constant of salt of weak acid and strong base.
🟦 For CH₃COONa: CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻.
🟨 Hydrolysis constant Kh = Kw/Ka.
🟩 pH > 7.
🧭 Section D (Q29–Q30) – Case-Based Questions (4 marks each, 2 × 4 = 8 marks)
Q29. Read the passage and answer the questions:
In the synthesis of ammonia (N₂ + 3H₂ ⇌ 2NH₃), the reaction is exothermic. According to Le Chatelier’s principle, changes in pressure, temperature, and concentration affect equilibrium.
(a) What is the effect of increasing pressure on the equilibrium? (1 mark)
(b) What is the effect of increasing temperature on equilibrium yield? (1 mark)
(c) Explain why high pressure and moderate temperature are used industrially. (2 marks)
🧪 Answer:
(a) High pressure shifts equilibrium towards ammonia (fewer gas molecules).
(b) High temperature shifts equilibrium backward (exothermic reaction).
(c) Industrially, high pressure increases yield, and moderate temperature ensures a balance of good yield and fast rate.
Q30. Read the passage and answer the questions:
The dissociation of acetic acid is suppressed by adding sodium acetate. This is the common-ion effect and is applied in buffer preparation.
(a) Write dissociation equation of acetic acid. (1 mark)
(b) What is the effect of adding CH₃COONa? (1 mark)
(c) Explain how buffer solution resists change in pH. (2 marks)
🧪 Answer:
(a) CH₃COOH ⇌ CH₃COO⁻ + H⁺.
(b) Addition of CH₃COONa increases [CH₃COO⁻], suppressing dissociation.
(c) Added H⁺ combines with CH₃COO⁻, and added OH⁻ reacts with CH₃COOH → pH remains nearly constant.
⚡ Section E (Q31–Q33) – Long Answer (5 marks each, 3 × 5 = 15 marks)
Q31. (a) State Le Chatelier’s principle. Apply it to the reaction N₂O₄ ⇌ 2NO₂ with respect to pressure and temperature changes.
OR
(b) Derive the relationship between Kp and Kc for a general gaseous reaction.
🧪 Answer (a):
🟦 Principle: If a system at equilibrium is disturbed, it shifts in a direction to counteract the change.
🟨 Pressure: Increase in pressure shifts equilibrium towards fewer gas molecules (N₂O₄).
🟩 Temperature: Since dissociation N₂O₄ → 2NO₂ is endothermic, higher T favors NO₂ formation.
Answer (b):
🟦 General reaction: aA + bB ⇌ cC + dD.
🟨 Kp = (pC^c pD^d)/(pA^a pB^b).
🟩 Using p = CRT → Kp = Kc(RT)^Δn, where Δn = (c + d – a – b).
Q32. (a) Define ionic product of water. Derive relation between Kw and pH.
OR
(b) Calculate pH of 0.01 M Ba(OH)₂ solution at 25 °C.
🧪 Answer (a):
🟦 Ionic product: Kw = [H⁺][OH⁻] = 1 × 10⁻¹⁴ at 25 °C.
🟨 In neutral water: [H⁺] = [OH⁻] = 1 × 10⁻⁷ M.
🟩 pH = –log[H⁺].
🎯 Relation: pKw = pH + pOH = 14.
Answer (b):
➤ Ba(OH)₂ → Ba²⁺ + 2OH⁻.
➤ [OH⁻] = 2 × 0.01 = 0.02 M.
➤ pOH = –log(0.02) ≈ 1.70.
➤ pH = 14 – 1.70 = 12.30.
✅ Final Answer: pH = 12.3.
Q33. (a) Explain buffer solution and derive Henderson–Hasselbalch equation.
OR
(b) Write notes on solubility product (Ksp). Derive expression for molar solubility of AgCl.
🧪 Answer (a):
🟦 Buffer: Resists pH change on addition of small acid/base.
🟨 Weak acid buffer: pH = pKa + log([salt]/[acid]).
🟩 Derivation: From Ka = [H⁺][A⁻]/[HA], rearranging gives Henderson equation.
🎯 Example: CH₃COOH/CH₃COONa buffer.
Answer (b):
🟦 Solubility product: Ksp = product of ionic concentrations of a sparingly soluble salt at equilibrium.
🟨 For AgCl ⇌ Ag⁺ + Cl⁻, Ksp = [Ag⁺][Cl⁻].
🟩 Let solubility = S → [Ag⁺] = [Cl⁻] = S.
🧮 Ksp = S² → S = √Ksp.
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
🔵 Question 1:
Which of the following is not a colligative property?
🔴 ① Relative lowering of vapour pressure
🟢 ② Osmotic pressure
🟡 ③ Boiling point elevation
🔵 ④ Viscosity
🟢 Answer: ④ Viscosity
📘 Exam: AIPMT
📅 Year: 2006 | Set: B
🔵 Question 2:
Which one of the following electrolytes has maximum coagulating power for +ve sol?
🔴 ① NaCl
🟢 ② CaCl₂
🟡 ③ AlCl₃
🔵 ④ KCl
🟢 Answer: ③ AlCl₃
📘 Exam: NEET
📅 Year: 2013 | Set: O
🔵 Question 3:
If the solubility product of Ag₂CrO₄ is 1.1 × 10⁻¹², the molar solubility of Ag₂CrO₄ is:
🔴 ① 1.1 × 10⁻⁴ M
🟢 ② 1.0 × 10⁻⁴ M
🟡 ③ 3.0 × 10⁻⁴ M
🔵 ④ 2.0 × 10⁻⁴ M
🟢 Answer: ② 1.0 × 10⁻⁴ M
📘 Exam: NEET
📅 Year: 2019 | Set: P2
🔵 Question 4:
pH of 0.001 M HCl solution is:
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 11
🟢 Answer: ③ 3
📘 Exam: AIPMT
📅 Year: 2005 | Set: A
🔵 Question 5:
The dissociation constant of a weak acid is 1.6 × 10⁻⁵. The degree of dissociation of the acid in 0.1 M solution is:
🔴 ① 0.04
🟢 ② 0.0126
🟡 ③ 0.016
🔵 ④ 0.0016
🟢 Answer: ② 0.0126
📘 Exam: NEET
📅 Year: 2015 | Set: P
🔵 Question 6:
At 25°C, the ionic product of water is:
🔴 ① 1 × 10⁻¹²
🟢 ② 1 × 10⁻¹⁴
🟡 ③ 1 × 10⁻¹⁶
🔵 ④ 1 × 10⁻¹⁸
🟢 Answer: ② 1 × 10⁻¹⁴
📘 Exam: NEET
📅 Year: 2017 | Set: S1
🔵 Question 7:
For the reaction: N₂(g) + 3H₂(g) ⇌ 2NH₃(g),
if pressure is increased, the equilibrium will shift:
🔴 ① Towards N₂
🟢 ② Towards H₂
🟡 ③ Towards NH₃
🔵 ④ No change
🟢 Answer: ③ Towards NH₃
📘 Exam: AIPMT
📅 Year: 2002 | Set: C
🔵 Question 8:
Which indicator is used in the titration of a strong acid with a strong base?
🔴 ① Methyl orange
🟢 ② Phenolphthalein
🟡 ③ Both (1) and (2)
🔵 ④ None
🟢 Answer: ③ Both (1) and (2)
📘 Exam: NEET
📅 Year: 2018 | Set: O2
🔵 Question 9:
For a buffer solution, the pH is given by:
🔴 ① pH = pKa – log([salt]/[acid])
🟢 ② pH = pKa + log([salt]/[acid])
🟡 ③ pH = pKw – pOH
🔵 ④ pH = –log[H⁺]
🟢 Answer: ② pH = pKa + log([salt]/[acid])
📘 Exam: AIPMT
📅 Year: 2007 | Set: A
🔵 Question 10:
Which of the following is the conjugate base of HSO₄⁻?
🔴 ① SO₄²⁻
🟢 ② H₂SO₄
🟡 ③ H₃O⁺
🔵 ④ OH⁻
🟢 Answer: ① SO₄²⁻
📘 Exam: NEET
📅 Year: 2014 | Set: M
🔵 Question 11:
Which one is a Lewis acid?
🔴 ① NH₃
🟢 ② BF₃
🟡 ③ OH⁻
🔵 ④ F⁻
🟢 Answer: ② BF₃
📘 Exam: AIPMT
📅 Year: 2004 | Set: B
🔵 Question 12:
At equilibrium, which one is true?
🔴 ① Forward reaction stops
🟢 ② Backward reaction stops
🟡 ③ Forward rate = Backward rate
🔵 ④ Both reactions stop
🟢 Answer: ③ Forward rate = Backward rate
📘 Exam: NEET
📅 Year: 2019 | Set: Q1
🔵 Question 13:
For a reaction, ΔG = 0. This implies:
🔴 ① K = 1
🟢 ② K < 1 🟡 ③ K > 1
🔵 ④ ΔH = 0
🟢 Answer: ① K = 1
📘 Exam: AIPMT
📅 Year: 2006 | Set: C
🔵 Question 14:
The solubility product (Ksp) of a salt AB is 1 × 10⁻⁸. Its solubility is:
🔴 ① 1 × 10⁻² M
🟢 ② 1 × 10⁻⁴ M
🟡 ③ 1 × 10⁻⁸ M
🔵 ④ 1 × 10⁻¹⁶ M
🟢 Answer: ② 1 × 10⁻⁴ M
📘 Exam: NEET
📅 Year: 2012 | Set: Q
🔵 Question 15:
The common ion effect is observed in:
🔴 ① Mixture of NaCl and KCl
🟢 ② Mixture of CH₃COOH and CH₃COONa
🟡 ③ Mixture of H₂SO₄ and NaOH
🔵 ④ Mixture of HCl and NaOH
🟢 Answer: ② Mixture of CH₃COOH and CH₃COONa
📘 Exam: NEET
📅 Year: 2016 | Set: S2
🔵 Question 16:
Which of the following solutions has pH > 7?
🔴 ① 0.1 M NaCl
🟢 ② 0.1 M Na₂CO₃
🟡 ③ 0.1 M NH₄Cl
🔵 ④ 0.1 M CH₃COOH
🟢 Answer: ② 0.1 M Na₂CO₃
📘 Exam: AIPMT
📅 Year: 2008 | Set: B
🔵 Question 17:
If solubility of AgCl is s, then its Ksp is:
🔴 ① s
🟢 ② s²
🟡 ③ 2s
🔵 ④ 4s²
🟢 Answer: ② s²
📘 Exam: NEET
📅 Year: 2015 | Set: P
🔵 Question 18:
The pH of 0.1 M solution of NH₄OH (Kb = 1.77 × 10⁻⁵) is approximately:
🔴 ① 11.1
🟢 ② 10.1
🟡 ③ 9.1
🔵 ④ 12.1
🟢 Answer: ③ 9.1
📘 Exam: NEET
📅 Year: 2018 | Set: M
🔵 Question 19:
The equilibrium constant of a reaction is 10. What is ΔG° at 300 K? (R = 8.314 J mol⁻¹ K⁻¹)
🔴 ① –5740 J
🟢 ② –574 J
🟡 ③ –57.4 J
🔵 ④ –5.74 kJ
🟢 Answer: ④ –5.74 kJ
📘 Exam: AIPMT
📅 Year: 2003 | Set: A
🔵 Question 20:
Which of the following is a weak base?
🔴 ① NH₃
🟢 ② NaOH
🟡 ③ KOH
🔵 ④ Ca(OH)₂
🟢 Answer: ① NH₃
📘 Exam: NEET
📅 Year: 2014 | Set: Q
🔵 Question 21:
For which reaction, Δn = 0?
🔴 ① N₂(g) + 3H₂(g) → 2NH₃(g)
🟢 ② H₂(g) + Cl₂(g) → 2HCl(g)
🟡 ③ 2SO₂(g) + O₂(g) → 2SO₃(g)
🔵 ④ N₂O₄(g) → 2NO₂(g)
🟢 Answer: ② H₂(g) + Cl₂(g) → 2HCl(g)
📘 Exam: AIPMT
📅 Year: 2010 | Set: B
🔵 Question 22:
For a given weak acid HA, Ka = 1 × 10⁻⁵ and concentration = 0.1 M. What is the pH?
🔴 ① 2.0
🟢 ② 3.0
🟡 ③ 4.0
🔵 ④ 5.0
🟢 Answer: ② 3.0
📘 Exam: NEET
📅 Year: 2016 | Set: Q
🔵 Question 23:
In Le-Chatelier’s principle, when temperature is increased for an endothermic reaction, equilibrium shifts:
🔴 ① Backward
🟢 ② Forward
🟡 ③ No change
🔵 ④ Both directions
🟢 Answer: ② Forward
📘 Exam: AIPMT
📅 Year: 2009 | Set: C
🔵 Question 24:
The solubility of BaSO₄ in water is 2 × 10⁻³ mol L⁻¹. Its solubility product is:
🔴 ① 2 × 10⁻³
🟢 ② 4 × 10⁻⁶
🟡 ③ 2 × 10⁻⁶
🔵 ④ 4 × 10⁻³
🟢 Answer: ② 4 × 10⁻⁶
📘 Exam: NEET
📅 Year: 2017 | Set: O1
🔵 Question 25:
Which of the following statements is correct for a buffer solution?
🔴 ① It resists change in pH
🟢 ② Its pH is always 7
🟡 ③ It completely neutralises acids
🔵 ④ It has no conjugate base
🟢 Answer: ① It resists change in pH
📘 Exam: NEET
📅 Year: 2020 | Set: S
🔵 Question 26:
For the reaction: N₂O₄(g) ⇌ 2NO₂(g), colourless ⇌ brown.
On increasing temperature, equilibrium shifts:
🔴 ① Towards N₂O₄
🟢 ② Towards NO₂
🟡 ③ No change
🔵 ④ Both directions
🟢 Answer: ② Towards NO₂
📘 Exam: NEET
📅 Year: 2017 | Set: P1
🔵 Question 27:
Which one is the conjugate acid of NH₃?
🔴 ① NH₂⁻
🟢 ② NH₄⁺
🟡 ③ N₂H₄
🔵 ④ H⁺
🟢 Answer: ② NH₄⁺
📘 Exam: AIPMT
📅 Year: 2002 | Set: A
🔵 Question 28:
The degree of dissociation of weak electrolyte is:
🔴 ① Directly proportional to concentration
🟢 ② Inversely proportional to concentration
🟡 ③ Independent of concentration
🔵 ④ None
🟢 Answer: ② Inversely proportional to concentration
📘 Exam: NEET
📅 Year: 2016 | Set: M
🔵 Question 29:
The pH of 10⁻⁸ M HCl solution is:
🔴 ① 8
🟢 ② 7
🟡 ③ 6.96
🔵 ④ 3
🟢 Answer: ③ 6.96
📘 Exam: NEET
📅 Year: 2012 | Set: O
🔵 Question 30:
Which of the following statements is true about equilibrium constant (K)?
🔴 ① Depends on temperature
🟢 ② Depends on concentration
🟡 ③ Depends on pressure
🔵 ④ Depends on catalyst
🟢 Answer: ① Depends on temperature
📘 Exam: AIPMT
📅 Year: 2007 | Set: B
🔵 Question 31:
For a salt MX, if solubility is S mol L⁻¹, the Ksp is:
🔴 ① S
🟢 ② S²
🟡 ③ 2S
🔵 ④ 4S²
🟢 Answer: ② S²
📘 Exam: NEET
📅 Year: 2019 | Set: Q
🔵 Question 32:
At 25°C, pH of neutral solution is:
🔴 ① 6
🟢 ② 7
🟡 ③ 8
🔵 ④ 14
🟢 Answer: ② 7
📘 Exam: NEET
📅 Year: 2015 | Set: Q
🔵 Question 33:
Which one of the following is a Lewis base?
🔴 ① AlCl₃
🟢 ② NH₃
🟡 ③ BF₃
🔵 ④ Fe³⁺
🟢 Answer: ② NH₃
📘 Exam: AIPMT
📅 Year: 2004 | Set: C
🔵 Question 34:
pOH of 0.001 M NaOH solution is:
🔴 ① 11
🟢 ② 3
🟡 ③ 14
🔵 ④ 1
🟢 Answer: ② 3
📘 Exam: NEET
📅 Year: 2017 | Set: R1
🔵 Question 35:
The solubility product expression of CaF₂ is:
🔴 ① [Ca²⁺][F⁻]²
🟢 ② [Ca²⁺]²[F⁻]
🟡 ③ [Ca²⁺]³[F⁻]²
🔵 ④ [Ca²⁺][F⁻]
🟢 Answer: ① [Ca²⁺][F⁻]²
📘 Exam: AIPMT
📅 Year: 2006 | Set: A
🔵 Question 36:
If pH of solution is 4, then H⁺ ion concentration is:
🔴 ① 1 × 10⁻³ M
🟢 ② 1 × 10⁻⁴ M
🟡 ③ 1 × 10⁻⁵ M
🔵 ④ 4 M
🟢 Answer: ② 1 × 10⁻⁴ M
📘 Exam: NEET
📅 Year: 2014 | Set: S
🔵 Question 37:
Which salt will show highest pH in aqueous solution?
🔴 ① Na₂CO₃
🟢 ② NaCl
🟡 ③ NH₄Cl
🔵 ④ KNO₃
🟢 Answer: ① Na₂CO₃
📘 Exam: NEET
📅 Year: 2013 | Set: Q
🔵 Question 38:
In Le-Chatelier’s principle, addition of inert gas at constant volume:
🔴 ① Shifts equilibrium forward
🟢 ② Shifts equilibrium backward
🟡 ③ No change
🔵 ④ Shifts equilibrium both sides
🟢 Answer: ③ No change
📘 Exam: AIPMT
📅 Year: 2008 | Set: B
🔵 Question 39:
Which solution has maximum pH?
🔴 ① 0.1 M NaOH
🟢 ② 0.1 M NH₄OH
🟡 ③ 0.1 M NaCl
🔵 ④ 0.1 M CH₃COOH
🟢 Answer: ① 0.1 M NaOH
📘 Exam: NEET
📅 Year: 2019 | Set: M
🔵 Question 40:
pH of blood in human body is:
🔴 ① 7.0
🟢 ② 7.4
🟡 ③ 6.8
🔵 ④ 8.0
🟢 Answer: ② 7.4
📘 Exam: AIPMT
📅 Year: 2005 | Set: C
🔵 Question 41:
Which one of the following is amphoteric?
🔴 ① NaOH
🟢 ② ZnO
🟡 ③ MgO
🔵 ④ CaO
🟢 Answer: ② ZnO
📘 Exam: NEET
📅 Year: 2018 | Set: R
🔵 Question 42:
For a buffer solution of CH₃COOH and CH₃COONa, if both concentrations are equal, pH =
🔴 ① pKa + 1
🟢 ② pKa – 1
🟡 ③ pKa
🔵 ④ 14 – pKa
🟢 Answer: ③ pKa
📘 Exam: NEET
📅 Year: 2016 | Set: O1
🔵 Question 43:
Which of the following is a strong electrolyte?
🔴 ① CH₃COOH
🟢 ② NaOH
🟡 ③ NH₄OH
🔵 ④ HCN
🟢 Answer: ② NaOH
📘 Exam: AIPMT
📅 Year: 2003 | Set: B
🔵 Question 44:
Solubility of AgCl decreases in presence of NaCl due to:
🔴 ① Common ion effect
🟢 ② Hydrolysis
🟡 ③ Oxidation
🔵 ④ Reduction
🟢 Answer: ① Common ion effect
📘 Exam: NEET
📅 Year: 2012 | Set: P
🔵 Question 45:
The ionisation constant of NH₄OH is 1.77 × 10⁻⁵. Its pKb is:
🔴 ① 4.75
🟢 ② 5.75
🟡 ③ 3.75
🔵 ④ 6.75
🟢 Answer: ① 4.75
📘 Exam: AIPMT
📅 Year: 2009 | Set: C
🔵 Question 46:
The equilibrium constant of a reaction is 1 × 10⁵. Then ΔG° at 300 K is approximately:
🔴 ① –28.5 kJ
🟢 ② –11.4 kJ
🟡 ③ –57 kJ
🔵 ④ –5.7 kJ
🟢 Answer: ① –28.5 kJ
📘 Exam: NEET
📅 Year: 2014 | Set: Q
🔵 Question 47:
The conjugate acid of HCO₃⁻ is:
🔴 ① CO₃²⁻
🟢 ② H₂CO₃
🟡 ③ H₂O
🔵 ④ OH⁻
🟢 Answer: ② H₂CO₃
📘 Exam: AIPMT
📅 Year: 2007 | Set: A
🔵 Question 48:
Which of the following is correct for strong acid?
🔴 ① Completely dissociates
🟢 ② Partially dissociates
🟡 ③ Does not dissociate
🔵 ④ Forms buffer solution
🟢 Answer: ① Completely dissociates
📘 Exam: NEET
📅 Year: 2015 | Set: L
🔵 Question 49:
The ionic product of water at 25°C is:
🔴 ① 1 × 10⁻¹²
🟢 ② 1 × 10⁻¹⁴
🟡 ③ 1 × 10⁻¹⁶
🔵 ④ 1 × 10⁻¹⁸
🟢 Answer: ② 1 × 10⁻¹⁴
📘 Exam: AIPMT
📅 Year: 2001 | Set: B
🔵 Question 50:
At equilibrium:
🔴 ① ΔG = 0
🟢 ② ΔH = 0
🟡 ③ ΔS = 0
🔵 ④ ΔG < 0
🟢 Answer: ① ΔG = 0
📘 Exam: NEET
📅 Year: 2019 | Set: M
————————————————————————————————————————————————————————————————————————————
JEE MAINS QUESTIONS FROM THIS LESSON
🔵 Question 1
The pH of 10⁻⁸ M HCl solution is:
🔴 ① 8
🟢 ② 6.96
🟡 ③ 7.0
🔵 ④ 8.04
🟢 Answer: ② 6.96
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Morning
🔵 Question 2
For the reaction: N₂O₄ (g) ⇌ 2NO₂ (g), increase in pressure will:
🔴 ① Shift equilibrium towards NO₂
🟢 ② Shift equilibrium towards N₂O₄
🟡 ③ Not affect equilibrium
🔵 ④ Decompose completely
🟢 Answer: ② Shift equilibrium towards N₂O₄
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Evening
🔵 Question 3
At 25 °C, pH of 10⁻³ M H₂SO₄ is nearly:
🔴 ① 3
🟢 ② 2.7
🟡 ③ 2.0
🔵 ④ 1.7
🟢 Answer: ④ 1.7
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Morning
🔵 Question 4
The conjugate base of NH₃ is:
🔴 ① H⁺
🟢 ② NH₂⁻
🟡 ③ NH₄⁺
🔵 ④ OH⁻
🟢 Answer: ② NH₂⁻
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Evening
🔵 Question 5
The solubility product of AgCl at 25 °C is 1.6 × 10⁻¹⁰. The solubility is:
🔴 ① 1.26 × 10⁻⁵ M
🟢 ② 4 × 10⁻¹⁰ M
🟡 ③ 1.6 × 10⁻¹⁰ M
🔵 ④ 4 × 10⁻⁵ M
🟢 Answer: ① 1.26 × 10⁻⁵ M
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Morning
🔵 Question 6
For a reaction A + B ⇌ C + D, equilibrium constant Kc is 10⁻¹⁰. The reaction is:
🔴 ① Nearly complete
🟢 ② Negligible forward reaction
🟡 ③ Fast
🔵 ④ Quantitative
🟢 Answer: ② Negligible forward reaction
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Evening
🔵 Question 7
Which of the following aqueous solutions has highest pH?
🔴 ① 0.1 M HCl
🟢 ② 0.1 M NaOH
🟡 ③ 0.1 M CH₃COOH
🔵 ④ 0.1 M NH₄Cl
🟢 Answer: ② 0.1 M NaOH
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Morning
🔵 Question 8
The pH of 0.01 M NaOH solution is:
🔴 ① 12
🟢 ② 11
🟡 ③ 10
🔵 ④ 9
🟢 Answer: ① 12
📘 Exam: JEE Main
📅 Year: 2013 | Shift: Morning
🔵 Question 9
Which is the weakest acid?
🔴 ① HCl
🟢 ② HNO₃
🟡 ③ CH₃COOH
🔵 ④ H₂SO₄
🟢 Answer: ③ CH₃COOH
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Evening
🔵 Question 10
The degree of dissociation of a weak electrolyte is proportional to:
🔴 ① c
🟢 ② 1/c
🟡 ③ √c
🔵 ④ 1/√c
🟢 Answer: ④ 1/√c
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Morning
🔵 Question 11
When common ion is added, solubility of salt:
🔴 ① Increases
🟢 ② Decreases
🟡 ③ Remains same
🔵 ④ May increase
🟢 Answer: ② Decreases
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Morning
🔵 Question 12
The dissociation constant (Ka) of a weak acid is 1.8 × 10⁻⁵. The pKa is:
🔴 ① 2.74
🟢 ② 4.74
🟡 ③ 5.74
🔵 ④ 6.74
🟢 Answer: ② 4.74
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Evening
🔵 Question 13
The equilibrium constant for a reaction is 1 × 10⁻⁵ at 25 °C. The reaction is:
🔴 ① Spontaneous
🟢 ② Forward reaction negligible
🟡 ③ At equilibrium
🔵 ④ Very fast
🟢 Answer: ② Forward reaction negligible
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Evening
🔵 Question 14
At 25 °C, the ionic product of water (Kw) is:
🔴 ① 1 × 10⁻¹²
🟢 ② 1 × 10⁻¹⁴
🟡 ③ 1 × 10⁻¹⁶
🔵 ④ 1 × 10⁻⁷
🟢 Answer: ② 1 × 10⁻¹⁴
📘 Exam: JEE Main
📅 Year: 2013 | Shift: Evening
🔵 Question 15
Which of the following is a buffer solution?
🔴 ① NaOH + HCl
🟢 ② CH₃COOH + CH₃COONa
🟡 ③ NaCl + HCl
🔵 ④ NH₄Cl + NaCl
🟢 Answer: ② CH₃COOH + CH₃COONa
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Morning
🔵 Question 16
The solubility of AgCl decreases in presence of:
🔴 ① NaCl
🟢 ② NaNO₃
🟡 ③ Na₂SO₄
🔵 ④ KNO₃
🟢 Answer: ① NaCl (common ion effect)
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Morning
🔵 Question 17
Which indicator is suitable for titration of strong acid vs strong base?
🔴 ① Phenolphthalein
🟢 ② Methyl orange
🟡 ③ Both
🔵 ④ None
🟢 Answer: ③ Both
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Evening
🔵 Question 18
The dissociation constant of acetic acid is 1.8 × 10⁻⁵. Its degree of dissociation at 0.1 M concentration is nearly:
🔴 ① 0.13
🟢 ② 0.042
🟡 ③ 0.0042
🔵 ④ 0.0013
🟢 Answer: ③ 0.0042
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Evening
🔵 Question 19
The pH of a 0.01 M HCl solution is:
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 4
🟢 Answer: ② 2
📘 Exam: JEE Main
📅 Year: 2013 | Shift: Morning
🔵 Question 20
In an acidic buffer, pH is given by:
🔴 ① pKa – log([Salt]/[Acid])
🟢 ② pKa + log([Salt]/[Acid])
🟡 ③ –log[H⁺]
🔵 ④ –log[OH⁻]
🟢 Answer: ② pKa + log([Salt]/[Acid])
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Evening
🔵 Question 21
When pressure is increased, solubility of a gas in liquid:
🔴 ① Decreases
🟢 ② Increases
🟡 ③ Remains same
🔵 ④ None
🟢 Answer: ② Increases (Henry’s law)
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Evening
🔵 Question 22
For dissociation of PCl₅ ⇌ PCl₃ + Cl₂, addition of PCl₃ will:
🔴 ① Shift forward
🟢 ② Shift backward
🟡 ③ No change
🔵 ④ Decompose
🟢 Answer: ② Shift backward
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Morning
🔵 Question 23
For a weak base BOH, Kb = 10⁻⁵. The pKb is:
🔴 ① 5
🟢 ② 4
🟡 ③ 3
🔵 ④ 2
🟢 Answer: ① 5
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Morning
🔵 Question 24
The Henderson–Hasselbalch equation is used in calculating:
🔴 ① Solubility
🟢 ② pH of buffer
🟡 ③ Kw
🔵 ④ Ionic strength
🟢 Answer: ② pH of buffer
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Evening
🔵 Question 25
Which of the following salts hydrolyses to give basic solution?
🔴 ① NaCl
🟢 ② CH₃COONa
🟡 ③ NH₄Cl
🔵 ④ KNO₃
🟢 Answer: ② CH₃COONa
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Morning
🔵 Question 26
For the reaction: 2SO₂(g) + O₂(g) ⇌ 2SO₃(g), the addition of SO₂ will:
🔴 ① Shift equilibrium forward
🟢 ② Shift equilibrium backward
🟡 ③ No change
🔵 ④ Stop the reaction
🟢 Answer: ① Shift equilibrium forward
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Morning
🔵 Question 27
The solubility product (Ksp) of BaSO₄ is 1 × 10⁻¹⁰ at 25 °C. Its solubility is:
🔴 ① 1 × 10⁻¹⁰ M
🟢 ② 1 × 10⁻⁵ M
🟡 ③ 1 × 10⁻² M
🔵 ④ 1 × 10⁻⁸ M
🟢 Answer: ② 1 × 10⁻⁵ M
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Morning
🔵 Question 28
At 25 °C, pOH of 0.001 M NaOH solution is:
🔴 ① 3
🟢 ② 11
🟡 ③ 12
🔵 ④ 2
🟢 Answer: ① 3
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Evening
🔵 Question 29
For NH₄OH ⇌ NH₄⁺ + OH⁻, if degree of dissociation is α and initial concentration is c, then Kb is:
🔴 ① cα²
🟢 ② cα
🟡 ③ α²/c
🔵 ④ c/α
🟢 Answer: ① cα²
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Evening
🔵 Question 30
Which solution has highest freezing point?
🔴 ① 0.1 M NaCl
🟢 ② 0.1 M MgCl₂
🟡 ③ 0.1 M Glucose
🔵 ④ 0.1 M Al₂(SO₄)₃
🟢 Answer: ③ 0.1 M Glucose (non-electrolyte)
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Morning
🔵 Question 31
The solubility product of Al(OH)₃ is Ksp. Its solubility is:
🔴 ① (Ksp)¹/²
🟢 ② (Ksp)¹/³
🟡 ③ (Ksp/27)¹/⁴
🔵 ④ (Ksp/9)¹/³
🟢 Answer: ③ (Ksp/27)¹/⁴
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Evening
🔵 Question 32
For a weak acid HA, pH is 4 and concentration is 0.1 M. The Ka is:
🔴 ① 1 × 10⁻³
🟢 ② 1 × 10⁻⁵
🟡 ③ 1 × 10⁻⁷
🔵 ④ 1 × 10⁻²
🟢 Answer: ② 1 × 10⁻⁵
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Morning
🔵 Question 33
Which of the following is correct for strong electrolytes?
🔴 ① Completely dissociated
🟢 ② Partially dissociated
🟡 ③ Never dissociated
🔵 ④ Depends on concentration
🟢 Answer: ① Completely dissociated
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Evening
🔵 Question 34
At equilibrium:
🔴 ① Forward and backward reactions stop
🟢 ② Rates of forward and backward reactions equal
🟡 ③ Kp = Kc always
🔵 ④ Reaction becomes irreversible
🟢 Answer: ② Rates of forward and backward reactions equal
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Evening
🔵 Question 35
The solubility of CaF₂ in water is S. Its Ksp is:
🔴 ① S²
🟢 ② 4S³
🟡 ③ S³
🔵 ④ 2S³
🟢 Answer: ② 4S³
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Morning
🔵 Question 36
The hydrolysis constant Kh of salt of weak acid and strong base is related to Kw and Ka as:
🔴 ① Kh = Kw/Ka
🟢 ② Kh = Ka/Kw
🟡 ③ Kh = √KwKa
🔵 ④ Kh = Kw × Ka
🟢 Answer: ① Kh = Kw/Ka
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Morning
🔵 Question 37
The common ion effect is observed in:
🔴 ① AgCl + NaNO₃
🟢 ② AgCl + NaCl
🟡 ③ BaSO₄ + Na₂SO₃
🔵 ④ NaCl + KNO₃
🟢 Answer: ② AgCl + NaCl
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Morning
🔵 Question 38
The degree of dissociation of a weak acid increases if:
🔴 ① Concentration increases
🟢 ② Concentration decreases
🟡 ③ Temperature decreases
🔵 ④ Common ion added
🟢 Answer: ② Concentration decreases
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Morning
🔵 Question 39
Which salt solution is acidic?
🔴 ① Na₂CO₃
🟢 ② NH₄Cl
🟡 ③ CH₃COONa
🔵 ④ NaCl
🟢 Answer: ② NH₄Cl
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Morning
🔵 Question 40
The ionic product of water (Kw) increases with:
🔴 ① Increase in temperature
🟢 ② Decrease in temperature
🟡 ③ Pressure increase
🔵 ④ Addition of salts
🟢 Answer: ① Increase in temperature
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Evening
🔵 Question 41
The solubility of Mg(OH)₂ in water is S. Its Ksp is:
🔴 ① S²
🟢 ② 2S³
🟡 ③ 4S³
🔵 ④ S³
🟢 Answer: ② 2S³
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Evening
🔵 Question 42
The pH of 10⁻⁶ M HCl is:
🔴 ① 6
🟢 ② 7
🟡 ③ 5.96
🔵 ④ 8
🟢 Answer: ③ 5.96
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Evening
🔵 Question 43
For hydrolysis of salt of weak base and strong acid, pH is:
🔴 ① < 7 🟢 ② > 7
🟡 ③ = 7
🔵 ④ Depends on conc.
🟢 Answer: ① < 7
📘 Exam: JEE Main
📅 Year: 2014 | Shift: Evening
🔵 Question 44
If reaction quotient Q > K, then:
🔴 ① Reaction proceeds forward
🟢 ② Reaction proceeds backward
🟡 ③ Reaction at equilibrium
🔵 ④ Reaction stops
🟢 Answer: ② Reaction proceeds backward
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Morning
🔵 Question 45
For CH₃COOH + H₂O ⇌ H₃O⁺ + CH₃COO⁻, Ka is given as:
🔴 ① [H⁺][CH₃COO⁻]/[CH₃COOH]
🟢 ② [CH₃COOH]/[H⁺][CH₃COO⁻]
🟡 ③ [H⁺]/[CH₃COOH]
🔵 ④ [CH₃COO⁻]/[CH₃COOH]
🟢 Answer: ① [H⁺][CH₃COO⁻]/[CH₃COOH]
📘 Exam: JEE Main
📅 Year: 2013 | Shift: Evening
🔵 Question 46
The solubility of salt AxBy is S. Its Ksp is:
🔴 ① S^(x+y)
🟢 ② (Sx)^x (Sy)^y
🟡 ③ (S^x)(S^y)
🔵 ④ [S^(x+y)]
🟢 Answer: ① S^(x+y) (stoichiometric relation)
📘 Exam: JEE Main
📅 Year: 2018 | Shift: Morning
🔵 Question 47
At 25 °C, if Kw = 1 × 10⁻¹⁴, then pH of neutral solution is:
🔴 ① 6
🟢 ② 7
🟡 ③ 8
🔵 ④ 14
🟢 Answer: ② 7
📘 Exam: JEE Main
📅 Year: 2019 | Shift: Morning
🔵 Question 48
In acidic buffer, addition of small amount of strong acid:
🔴 ① Increases pH sharply
🟢 ② Decreases pH slightly
🟡 ③ No effect
🔵 ④ Increases pH slightly
🟢 Answer: ② Decreases pH slightly
📘 Exam: JEE Main
📅 Year: 2016 | Shift: Evening
🔵 Question 49
If solubility of AgCl is 1.26 × 10⁻⁵ M, its Ksp is:
🔴 ① 1.6 × 10⁻¹⁰
🟢 ② 1.6 × 10⁻⁵
🟡 ③ 1.6 × 10⁻²
🔵 ④ 1.6 × 10⁻¹²
🟢 Answer: ① 1.6 × 10⁻¹⁰
📘 Exam: JEE Main
📅 Year: 2015 | Shift: Morning
🔵 Question 50
For equilibrium: 2NOCl(g) ⇌ 2NO(g) + Cl₂(g), if volume increases:
🔴 ① Forward reaction favoured
🟢 ② Backward reaction favoured
🟡 ③ No effect
🔵 ④ Reaction stops
🟢 Answer: ① Forward reaction favoured (↑ no. of moles)
📘 Exam: JEE Main
📅 Year: 2017 | Shift: Evening
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
🔵 Question 1:
For the equilibrium, N₂O₄(g) ⇌ 2NO₂(g), the value of Kp is 0.66 at 400 K. If equilibrium pressure is 2 atm, the degree of dissociation of N₂O₄ is:
🔴 ① 0.20
🟢 ② 0.33
🟡 ③ 0.50
🔵 ④ 0.66
🟢 Answer: ② 0.33
📘 Exam: JEE Advanced
📅 Year: 2010 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 2:
In the dissociation of PCl₅, PCl₅ ⇌ PCl₃ + Cl₂, the degree of dissociation is 0.4 at equilibrium. The ratio of equilibrium pressure to the initial pressure is:
🔴 ① 1.0
🟢 ② 1.2
🟡 ③ 1.4
🔵 ④ 1.8
🟢 Answer: ③ 1.4
📘 Exam: JEE Advanced
📅 Year: 2013 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 3:
For a chemical equilibrium, ΔG is related to equilibrium constant K by:
🔴 ① ΔG = RT ln K
🟢 ② ΔG° = –RT ln K
🟡 ③ ΔG = –RT ln Kp
🔵 ④ ΔH = RT ln K
🟢 Answer: ② ΔG° = –RT ln K
📘 Exam: JEE Advanced
📅 Year: 2011 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 4:
The equilibrium constant of the reaction 2HI ⇌ H₂ + I₂ is 0.0156 at 298 K. The reaction is:
🔴 ① Product-favoured
🟢 ② Reactant-favoured
🟡 ③ At equilibrium with equal concentration
🔵 ④ Fast reaction
🟢 Answer: ② Reactant-favoured
📘 Exam: JEE Advanced
📅 Year: 2007 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 5:
For a reaction, A ⇌ B, the equilibrium constant is 10. The ratio of concentration of B to A at equilibrium is:
🔴 ① 1 : 10
🟢 ② 10 : 1
🟡 ③ 1 : 1
🔵 ④ 100 : 1
🟢 Answer: ② 10 : 1
📘 Exam: JEE Advanced
📅 Year: 2012 | Paper: 1 | Conducted by: IIT Delhi
🔵 Question 6:
The pH of a 0.1 M solution of weak acid HA is 3.0. The dissociation constant (Ka) is:
🔴 ① 1 × 10⁻³
🟢 ② 1 × 10⁻⁵
🟡 ③ 1 × 10⁻⁷
🔵 ④ 1 × 10⁻⁹
🟢 Answer: ② 1 × 10⁻⁵
📘 Exam: JEE Advanced
📅 Year: 2015 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 7:
In a buffer solution of acetic acid and sodium acetate, the pH is given by:
🔴 ① pH = pKa – log [salt]/[acid]
🟢 ② pH = pKa + log [salt]/[acid]
🟡 ③ pH = pKw – pKa
🔵 ④ pH = pKa – pKw
🟢 Answer: ② pH = pKa + log [salt]/[acid]
📘 Exam: JEE Advanced
📅 Year: 2014 | Paper: 1 | Conducted by: IIT Kanpur
🔵 Question 8:
Which of the following salts will hydrolyse in water to give an acidic solution?
🔴 ① NaCl
🟢 ② CH₃COONa
🟡 ③ NH₄Cl
🔵 ④ KNO₃
🟢 Answer: ③ NH₄Cl
📘 Exam: JEE Advanced
📅 Year: 2009 | Paper: 1 | Conducted by: IIT Roorkee
🔵 Question 9:
For a reaction, A + B ⇌ C + D, if the concentration of C is doubled, the equilibrium will shift:
🔴 ① Towards right
🟢 ② Towards left
🟡 ③ No change
🔵 ④ Rate increases
🟢 Answer: ② Towards left
📘 Exam: JEE Advanced
📅 Year: 2016 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 10:
The ionisation constant of formic acid is 1.8 × 10⁻⁴. The degree of dissociation of 0.1 M formic acid is:
🔴 ① 0.134
🟢 ② 0.042
🟡 ③ 0.018
🔵 ④ 0.0042
🟢 Answer: ② 0.042
📘 Exam: JEE Advanced
📅 Year: 2011 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 11:
The pH of 10⁻⁸ M HCl solution is closest to:
🔴 ① 8
🟢 ② 7
🟡 ③ 6.96
🔵 ④ 6
🟢 Answer: ③ 6.96
📘 Exam: JEE Advanced
📅 Year: 2012 | Paper: 1 | Conducted by: IIT Delhi
🔵 Question 12:
The common ion effect is observed in which of the following?
🔴 ① CH₃COOH + NaCl
🟢 ② CH₃COOH + CH₃COONa
🟡 ③ NH₄Cl + NaOH
🔵 ④ HCl + NaOH
🟢 Answer: ② CH₃COOH + CH₃COONa
📘 Exam: JEE Advanced
📅 Year: 2017 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 13:
The equilibrium constant Kc for a reaction is 1 × 10⁻¹⁰. What does it indicate?
🔴 ① Products are favoured
🟢 ② Reactants are favoured
🟡 ③ Both equally favoured
🔵 ④ Reaction is fast
🟢 Answer: ② Reactants are favoured
📘 Exam: JEE Advanced
📅 Year: 2014 | Paper: 1 | Conducted by: IIT Kanpur
🔵 Question 14:
The solubility product of AgCl is 1.6 × 10⁻¹⁰. Its solubility in water is:
🔴 ① 1.6 × 10⁻⁵ M
🟢 ② 4.0 × 10⁻⁵ M
🟡 ③ 1.2 × 10⁻⁵ M
🔵 ④ 2.5 × 10⁻⁵ M
🟢 Answer: ① 1.6 × 10⁻⁵ M
📘 Exam: JEE Advanced
📅 Year: 2008 | Paper: 1 | Conducted by: IIT Roorkee
🔵 Question 15:
If Qc > Kc for a given reaction, the reaction will proceed:
🔴 ① Forward
🟢 ② Backward
🟡 ③ Both directions equally
🔵 ④ Stop
🟢 Answer: ② Backward
📘 Exam: JEE Advanced
📅 Year: 2016 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 16:
Which one of the following solutions has the highest pH?
🔴 ① 0.1 M HCl
🟢 ② 0.1 M NaOH
🟡 ③ 0.1 M NH₄Cl
🔵 ④ 0.1 M CH₃COOH
🟢 Answer: ② 0.1 M NaOH
📘 Exam: JEE Advanced
📅 Year: 2015 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 17:
The Le Chatelier’s principle is not applicable to:
🔴 ① Physical equilibria
🟢 ② Chemical equilibria
🟡 ③ Equilibria involving change of temperature
🔵 ④ Equilibria involving catalysts
🟢 Answer: ④ Equilibria involving catalysts
📘 Exam: JEE Advanced
📅 Year: 2011 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 18:
Which of the following aqueous solutions has the lowest pH?
🔴 ① 0.1 M HCl
🟢 ② 0.1 M CH₃COOH
🟡 ③ 0.1 M NaOH
🔵 ④ 0.1 M NH₃
🟢 Answer: ① 0.1 M HCl
📘 Exam: JEE Advanced
📅 Year: 2012 | Paper: 1 | Conducted by: IIT Delhi
🔵 Question 19:
At 298 K, the equilibrium constant (K) for the reaction, N₂ + O₂ ⇌ 2NO is 4 × 10⁻³¹. What is ΔG° for the reaction? (R = 8.314 J mol⁻¹ K⁻¹)
🔴 ① +175 kJ mol⁻¹
🟢 ② –175 kJ mol⁻¹
🟡 ③ +85 kJ mol⁻¹
🔵 ④ –85 kJ mol⁻¹
🟢 Answer: ① +175 kJ mol⁻¹
📘 Exam: JEE Advanced
📅 Year: 2014 | Paper: 1 | Conducted by: IIT Kanpur
🔵 Question 20:
The pH of 0.001 M HCl solution is:
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 11
🟢 Answer: ③ 3
📘 Exam: JEE Advanced
📅 Year: 2010 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 21:
In the equilibrium, 2SO₂(g) + O₂(g) ⇌ 2SO₃(g), addition of O₂ will:
🔴 ① Shift equilibrium to left
🟢 ② Shift equilibrium to right
🟡 ③ Not affect equilibrium
🔵 ④ Stop reaction
🟢 Answer: ② Shift equilibrium to right
📘 Exam: JEE Advanced
📅 Year: 2007 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 22:
Which of the following will not affect the equilibrium constant of a chemical reaction?
🔴 ① Temperature
🟢 ② Pressure
🟡 ③ Concentration
🔵 ④ Catalyst
🟢 Answer: ④ Catalyst
📘 Exam: JEE Advanced
📅 Year: 2016 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 23:
The ionic product of water (Kw) at 25 °C is:
🔴 ① 1 × 10⁻¹⁴
🟢 ② 1 × 10⁻¹²
🟡 ③ 1 × 10⁻¹⁶
🔵 ④ 1 × 10⁻¹⁰
🟢 Answer: ① 1 × 10⁻¹⁴
📘 Exam: JEE Advanced
📅 Year: 2008 | Paper: 1 | Conducted by: IIT Roorkee
🔵 Question 24:
The solubility of AgCl in 0.1 M NaCl solution is less than its solubility in pure water due to:
🔴 ① Hydrolysis
🟢 ② Common ion effect
🟡 ③ Temperature effect
🔵 ④ Oxidation
🟢 Answer: ② Common ion effect
📘 Exam: JEE Advanced
📅 Year: 2015 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 25:
At 298 K, ΔG° for a reaction is zero. This implies:
🔴 ① K = 0
🟢 ② K = 1
🟡 ③ K < 1 🔵 ④ K > 1
🟢 Answer: ② K = 1
📘 Exam: JEE Advanced
📅 Year: 2013 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 26:
For a reaction, A ⇌ B, the forward and backward rate constants are equal. The value of equilibrium constant K is:
🔴 ① 0
🟢 ② 1
🟡 ③ < 1 🔵 ④ > 1
🟢 Answer: ② 1
📘 Exam: JEE Advanced
📅 Year: 2017 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 27:
The pH of 0.01 M NaOH solution is:
🔴 ① 2
🟢 ② 12
🟡 ③ 14
🔵 ④ 10
🟢 Answer: ② 12
📘 Exam: JEE Advanced
📅 Year: 2011 | Paper: 1 | Conducted by: IIT Madras
🔵 Question 28:
The dissociation constant (Ka) of acetic acid is 1.8 × 10⁻⁵. The degree of dissociation of 0.01 M CH₃COOH is:
🔴 ① 0.042
🟢 ② 0.134
🟡 ③ 0.018
🔵 ④ 0.0042
🟢 Answer: ① 0.042
📘 Exam: JEE Advanced
📅 Year: 2010 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 29:
Which of the following will hydrolyse to give a basic solution?
🔴 ① NaCl
🟢 ② CH₃COONa
🟡 ③ NH₄Cl
🔵 ④ AlCl₃
🟢 Answer: ② CH₃COONa
📘 Exam: JEE Advanced
📅 Year: 2016 | Paper: 1 | Conducted by: IIT Guwahati
🔵 Question 30:
For a saturated solution of a sparingly soluble salt AB, the solubility product (Ksp) is equal to:
🔴 ① [AB]
🟢 ② [A⁺][B⁻]
🟡 ③ [AB]²
🔵 ④ [A⁺]²[B⁻]²
🟢 Answer: ② [A⁺][B⁻]
📘 Exam: JEE Advanced
📅 Year: 2014 | Paper: 1 | Conducted by: IIT Kanpur
🔵 Question 31:
At equilibrium:
🔴 ① Forward rate = Backward rate
🟢 ② Forward rate > Backward rate
🟡 ③ Forward rate < Backward rate
🔵 ④ Concentration of reactants = Concentration of products
🟢 Answer: ① Forward rate = Backward rate
📘 Exam: JEE Advanced
📅 Year: 2009 | Paper: 1 | Conducted by: IIT Roorkee
🔵 Question 32:
The Henderson–Hasselbalch equation is used in calculating:
🔴 ① Heat of reaction
🟢 ② pH of buffer solutions
🟡 ③ Solubility product
🔵 ④ Ionic product of water
🟢 Answer: ② pH of buffer solutions
📘 Exam: JEE Advanced
📅 Year: 2018 | Paper: 1 | Conducted by: IIT Kanpur
🔵 Question 33:
Which of the following is a Lewis acid?
🔴 ① NH₃
🟢 ② AlCl₃
🟡 ③ OH⁻
🔵 ④ Cl⁻
🟢 Answer: ② AlCl₃
📘 Exam: JEE Advanced
📅 Year: 2007 | Paper: 1 | Conducted by: IIT Bombay
🔵 Question 34:
For a salt of weak base and strong acid, the aqueous solution will be:
🔴 ① Neutral
🟢 ② Acidic
🟡 ③ Basic
🔵 ④ Amphoteric
🟢 Answer: ② Acidic
📘 Exam: JEE Advanced
📅 Year: 2012 | Paper: 1 | Conducted by: IIT Delhi
————————————————————————————————————————————————————————————————————————————
PRACTICE SETS FROM THIS LESSON
🔵 Question 1:
The branch of chemistry which deals with energy changes during chemical reactions is:
🔴 ① Electrochemistry
🟢 ② Thermodynamics
🟡 ③ Chemical kinetics
🔵 ④ Photochemistry
🟢 Answer: ② Thermodynamics
🎯 Difficulty: NEET
🔵 Question 2:
The first law of thermodynamics is a statement of:
🔴 ① Conservation of mass
🟢 ② Conservation of energy
🟡 ③ Conservation of momentum
🔵 ④ Conservation of entropy
🟢 Answer: ② Conservation of energy
🎯 Difficulty: NEET
🔵 Question 3:
The SI unit of heat is:
🔴 ① Calorie
🟢 ② Joule
🟡 ③ Erg
🔵 ④ Kilocalorie
🟢 Answer: ② Joule
🎯 Difficulty: NEET
🔵 Question 4:
Internal energy is a:
🔴 ① Path function
🟢 ② State function
🟡 ③ Work function
🔵 ④ None
🟢 Answer: ② State function
🎯 Difficulty: NEET
🔵 Question 5:
The enthalpy of elements in their standard states is taken as:
🔴 ① Zero
🟢 ② One
🟡 ③ Positive
🔵 ④ Negative
🟢 Answer: ① Zero
🎯 Difficulty: NEET
🔵 Question 6:
Work done in isothermal reversible expansion of an ideal gas is given by:
🔴 ① W = nRT ln(V₂/V₁)
🟢 ② W = –PΔV
🟡 ③ W = ΔE
🔵 ④ W = q
🟢 Answer: ① W = nRT ln(V₂/V₁)
🎯 Difficulty: NEET
🔵 Question 7:
Which process occurs at constant pressure?
🔴 ① Isochoric
🟢 ② Isobaric
🟡 ③ Isothermal
🔵 ④ Adiabatic
🟢 Answer: ② Isobaric
🎯 Difficulty: NEET
🔵 Question 8:
The heat change at constant pressure is equal to change in:
🔴 ① Internal energy
🟢 ② Enthalpy
🟡 ③ Entropy
🔵 ④ Work
🟢 Answer: ② Enthalpy
🎯 Difficulty: NEET
🔵 Question 9:
Which one is an extensive property?
🔴 ① Pressure
🟢 ② Temperature
🟡 ③ Volume
🔵 ④ Density
🟢 Answer: ③ Volume
🎯 Difficulty: NEET
🔵 Question 10:
Which one is an intensive property?
🔴 ① Heat capacity
🟢 ② Enthalpy
🟡 ③ Density
🔵 ④ Internal energy
🟢 Answer: ③ Density
🎯 Difficulty: NEET
🔵 Question 11:
The amount of heat required to raise the temperature of 1 mol of a substance by 1 K is:
🔴 ① Specific heat
🟢 ② Molar heat capacity
🟡 ③ Latent heat
🔵 ④ Enthalpy
🟢 Answer: ② Molar heat capacity
🎯 Difficulty: NEET
🔵 Question 12:
In adiabatic process, exchange of heat is:
🔴 ① Maximum
🟢 ② Zero
🟡 ③ Minimum
🔵 ④ Positive
🟢 Answer: ② Zero
🎯 Difficulty: NEET
🔵 Question 13:
The entropy of a perfectly crystalline substance at absolute zero is:
🔴 ① Infinite
🟢 ② Zero
🟡 ③ One
🔵 ④ Cannot be defined
🟢 Answer: ② Zero
🎯 Difficulty: NEET
🔵 Question 14:
ΔG = ΔH – TΔS is the equation of:
🔴 ① First law of thermodynamics
🟢 ② Gibbs free energy
🟡 ③ Work function
🔵 ④ Helmholtz energy
🟢 Answer: ② Gibbs free energy
🎯 Difficulty: NEET
🔵 Question 15:
In an isothermal process of an ideal gas:
🔴 ① ΔE = 0
🟢 ② ΔH = 0
🟡 ③ ΔE = ΔH = 0
🔵 ④ None
🟢 Answer: ③ ΔE = ΔH = 0
🎯 Difficulty: NEET
🔵 Question 16:
Which law is also called the “law of constant heat summation”?
🔴 ① Hess’s law
🟢 ② First law of thermodynamics
🟡 ③ Zeroth law
🔵 ④ Second law
🟢 Answer: ① Hess’s law
🎯 Difficulty: NEET
🔵 Question 17:
Which property determines the spontaneity of a process?
🔴 ① ΔH
🟢 ② ΔG
🟡 ③ ΔS
🔵 ④ ΔE
🟢 Answer: ② ΔG
🎯 Difficulty: NEET
🔵 Question 18:
For a spontaneous reaction at constant T and P, ΔG is:
🔴 ① Positive
🟢 ② Negative
🟡 ③ Zero
🔵 ④ Infinite
🟢 Answer: ② Negative
🎯 Difficulty: NEET
🔵 Question 19:
Which one is a state function?
🔴 ① Work
🟢 ② Heat
🟡 ③ Enthalpy
🔵 ④ Path
🟢 Answer: ③ Enthalpy
🎯 Difficulty: NEET
🔵 Question 20:
The condition for equilibrium in terms of Gibbs free energy is:
🔴 ① ΔG > 0
🟢 ② ΔG = 0
🟡 ③ ΔG < 0
🔵 ④ ΔG = –TΔS
🟢 Answer: ② ΔG = 0
🎯 Difficulty: NEET
🔵 Question 21:
The latent heat of fusion of ice at 0°C is:
🔴 ① 3.36 kJ/mol
🟢 ② 6.01 kJ/mol
🟡 ③ 40.7 kJ/mol
🔵 ④ 1.0 kJ/mol
🟢 Answer: ② 6.01 kJ/mol
🎯 Difficulty: NEET
🔵 Question 22:
Which law of thermodynamics defines temperature?
🔴 ① Zeroth law
🟢 ② First law
🟡 ③ Second law
🔵 ④ Third law
🟢 Answer: ① Zeroth law
🎯 Difficulty: NEET
🔵 Question 23:
The heat absorbed by a system at constant volume is equal to:
🔴 ① ΔH
🟢 ② ΔE
🟡 ③ qₚ
🔵 ④ Work
🟢 Answer: ② ΔE
🎯 Difficulty: NEET
🔵 Question 24:
In which type of process ΔH = ΔE?
🔴 ① Isothermal
🟢 ② Isochoric
🟡 ③ Isobaric
🔵 ④ Adiabatic
🟢 Answer: ② Isochoric
🎯 Difficulty: NEET
🔵 Question 25:
The free expansion of an ideal gas is an example of:
🔴 ① Irreversible process
🟢 ② Reversible process
🟡 ③ Isothermal work
🔵 ④ Adiabatic reversible
🟢 Answer: ① Irreversible process
🎯 Difficulty: NEET
🔵 Question 26:
For an isothermal reversible expansion of an ideal gas, work done depends on:
🔴 ① Initial and final pressure/volume
🟢 ② Path followed
🟡 ③ Temperature change
🔵 ④ Heat capacity
🟢 Answer: ① Initial and final pressure/volume
🎯 Difficulty: JEE Main
🔵 Question 27:
If ΔH = –100 kJ and ΔS = –200 J/K, the reaction is spontaneous at:
🔴 ① Low temperature
🟢 ② High temperature
🟡 ③ All temperatures
🔵 ④ Never spontaneous
🟢 Answer: ① Low temperature
🎯 Difficulty: JEE Main
🔵 Question 28:
The maximum work done by a system in a reversible isothermal expansion is equal to:
🔴 ① ΔE
🟢 ② ΔH
🟡 ③ q
🔵 ④ ΔG
🟢 Answer: ④ ΔG
🎯 Difficulty: JEE Main
🔵 Question 29:
If ΔU = +20 J and W = –15 J, then q = ?
🔴 ① +35 J
🟢 ② +5 J
🟡 ③ –5 J
🔵 ④ –35 J
🟢 Answer: ② +5 J
🎯 Difficulty: JEE Main
🔵 Question 30:
The enthalpy change for the reaction C(graphite) → C(diamond) is positive. This indicates:
🔴 ① Diamond is more stable
🟢 ② Graphite is more stable
🟡 ③ Both equally stable
🔵 ④ None
🟢 Answer: ② Graphite is more stable
🎯 Difficulty: JEE Main
🔵 Question 31:
If ΔH = 50 kJ and ΔS = 100 J/K, at what minimum temperature will the reaction become spontaneous?
🔴 ① 100 K
🟢 ② 200 K
🟡 ③ 300 K
🔵 ④ 500 K
🟢 Answer: ② 200 K
🎯 Difficulty: JEE Main
🔵 Question 32:
In an isobaric process, heat supplied is used for:
🔴 ① Only increasing internal energy
🟢 ② Only work done
🟡 ③ Both ΔE and work
🔵 ④ Only ΔH
🟢 Answer: ③ Both ΔE and work
🎯 Difficulty: JEE Main
🔵 Question 33:
A reaction is endothermic and has positive entropy change. It will be:
🔴 ① Always spontaneous
🟢 ② Spontaneous at high T
🟡 ③ Spontaneous at low T
🔵 ④ Non-spontaneous
🟢 Answer: ② Spontaneous at high T
🎯 Difficulty: JEE Main
🔵 Question 34:
Which property is NOT a state function?
🔴 ① Heat
🟢 ② Enthalpy
🟡 ③ Internal energy
🔵 ④ Entropy
🟢 Answer: ① Heat
🎯 Difficulty: JEE Main
🔵 Question 35:
During an adiabatic reversible expansion of an ideal gas:
🔴 ① q = 0
🟢 ② ΔE = –W
🟡 ③ ΔH = ΔE
🔵 ④ All of these
🟢 Answer: ④ All of these
🎯 Difficulty: JEE Main
🔵 Question 36:
ΔU of a system can be calculated from:
🔴 ① q + W
🟢 ② q – W
🟡 ③ q/W
🔵 ④ None
🟢 Answer: ① q + W
🎯 Difficulty: JEE Main
🔵 Question 37:
The efficiency of a Carnot engine depends upon:
🔴 ① Nature of working substance
🟢 ② Temperature of source and sink
🟡 ③ Pressure of system
🔵 ④ Volume of system
🟢 Answer: ② Temperature of source and sink
🎯 Difficulty: JEE Main
🔵 Question 38:
Which process has ΔE = ΔH = 0?
🔴 ① Isothermal expansion of an ideal gas
🟢 ② Cooling of a solid
🟡 ③ Melting of ice
🔵 ④ Freezing of water
🟢 Answer: ① Isothermal expansion of an ideal gas
🎯 Difficulty: JEE Main
🔵 Question 39:
ΔH is less than ΔU when:
🔴 ① Reaction produces more moles of gases
🟢 ② Reaction consumes moles of gases
🟡 ③ No gases involved
🔵 ④ Reaction is exothermic
🟢 Answer: ② Reaction consumes moles of gases
🎯 Difficulty: JEE Main
🔵 Question 40:
In a cyclic process:
🔴 ① ΔE = 0
🟢 ② ΔH = 0
🟡 ③ ΔS = 0
🔵 ④ All of these
🟢 Answer: ④ All of these
🎯 Difficulty: JEE Main
🚀 Advanced-level (Q41–Q50):
🔵 Question 41:
If an ideal gas expands isothermally and reversibly, entropy change of the system is:
🔴 ① Zero
🟢 ② nR ln(V₂/V₁)
🟡 ③ ΔH/T
🔵 ④ ΔE/T
🟢 Answer: ② nR ln(V₂/V₁)
🎯 Difficulty: JEE Advanced
🔵 Question 42:
The efficiency of a Carnot engine operating between 500 K and 300 K is:
🔴 ① 0.2
🟢 ② 0.3
🟡 ③ 0.4
🔵 ④ 0.5
🟢 Answer: ③ 0.4
🎯 Difficulty: JEE Advanced
🔵 Question 43:
If ΔH = 100 kJ and ΔE = 80 kJ, the work done by the system at 300 K is:
🔴 ① 20 kJ
🟢 ② 100 kJ
🟡 ③ 80 kJ
🔵 ④ Zero
🟢 Answer: ① 20 kJ
🎯 Difficulty: JEE Advanced
🔵 Question 44:
Entropy is a measure of:
🔴 ① Heat content
🟢 ② Disorder of a system
🟡 ③ Internal energy
🔵 ④ Work function
🟢 Answer: ② Disorder of a system
🎯 Difficulty: JEE Advanced
🔵 Question 45:
For a spontaneous endothermic reaction:
🔴 ① ΔH < 0, ΔS < 0 🟢 ② ΔH > 0, ΔS > 0
🟡 ③ ΔH < 0, ΔS > 0
🔵 ④ ΔH > 0, ΔS < 0
🟢 Answer: ② ΔH > 0, ΔS > 0
🎯 Difficulty: JEE Advanced
🔵 Question 46:
The second law of thermodynamics introduces the concept of:
🔴 ① Enthalpy
🟢 ② Entropy
🟡 ③ Gibbs energy
🔵 ④ Internal energy
🟢 Answer: ② Entropy
🎯 Difficulty: JEE Advanced
🔵 Question 47:
If ΔS(universe) > 0, the process is:
🔴 ① Non-spontaneous
🟢 ② Spontaneous
🟡 ③ At equilibrium
🔵 ④ Impossible
🟢 Answer: ② Spontaneous
🎯 Difficulty: JEE Advanced
🔵 Question 48:
The entropy change in reversible adiabatic process is:
🔴 ① Maximum
🟢 ② Minimum
🟡 ③ Zero
🔵 ④ Infinite
🟢 Answer: ③ Zero
🎯 Difficulty: JEE Advanced
🔵 Question 49:
If one mole of an ideal gas expands isothermally at 300 K from 10 L to 20 L, the work done is (R = 8.314 J/mol·K):
🔴 ① 1.73 kJ
🟢 ② 1.73 J
🟡 ③ 17.3 J
🔵 ④ 173 J
🟢 Answer: ① 1.73 kJ
🎯 Difficulty: JEE Advanced
🔵 Question 50:
According to the third law of thermodynamics, entropy of a pure crystalline substance at absolute zero is:
🔴 ① Infinite
🟢 ② Zero
🟡 ③ One
🔵 ④ Cannot be defined
🟢 Answer: ② Zero
🎯 Difficulty: JEE Advanced
————————————————————————————————————————————————————————————————————————————
