Class : 9 – Science (English) : Lesson 2. Is Matter Around Us Pure?
EXPLANATION & SUMMARY
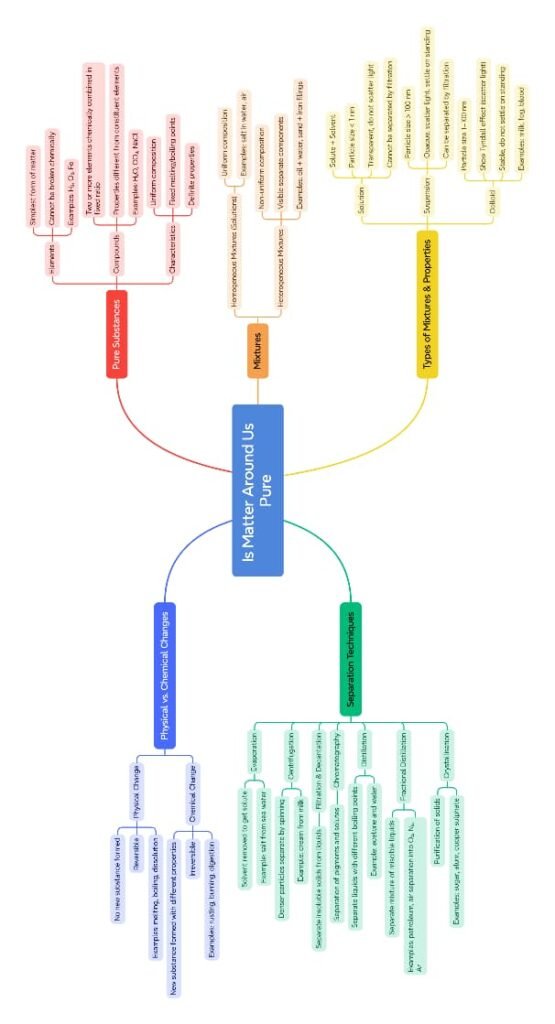
The lesson Is Matter Around Us Pure from Class 9 Science introduces students to the idea of chemical purity and classification of substances based on their composition. It begins by challenging the general use of the word “pure” in everyday life—such as pure milk or pure water—and contrasts it with its scientific meaning.
In science, a pure substance is one that contains only one kind of particle. These particles can be atoms or molecules, and the substance has a fixed composition. Pure substances are further classified into:
Elements: Made of only one kind of atom (e.g., hydrogen, carbon, iron).
Compounds: Made of two or more elements chemically combined in a fixed ratio (e.g., water – H₂O, carbon dioxide – CO₂). These have definite properties and can only be broken down by chemical methods.

On the other hand, a mixture consists of two or more substances that are physically combined, not chemically. Mixtures do not have a fixed composition and retain the properties of their components. Mixtures are divided into:
Homogeneous mixtures: These have uniform composition throughout. For example, sugar dissolved in water forms a solution where the particles are completely mixed.
Heterogeneous mixtures: These do not have a uniform composition, and different components are visible, such as a mixture of oil and water.
The chapter then explains different methods of separation used to isolate components of a mixture based on their physical properties:
Filtration: Separates an insoluble solid from a liquid.
Evaporation: Removes a liquid from a solution, leaving the solute behind (e.g., salt from seawater).
Centrifugation: Spins a mixture at high speed to separate denser particles (used in separating cream from milk).
Sublimation: Used to separate substances like camphor that change directly from solid to gas on heating.
Chromatography: Separates mixtures of dyes or pigments based on their ability to travel with a solvent.
Distillation: Separates liquids with different boiling points (used to purify water).
Fractional distillation: Separates a mixture of liquids with close boiling points (e.g., components of petroleum).
The lesson also introduces three types of mixtures based on particle size and behavior:
Solutions: Homogeneous mixtures where the solute dissolves completely in the solvent. Particles are too small to be seen or filtered and do not scatter light.
Suspensions: Heterogeneous mixtures where the solid particles do not dissolve and can settle down on standing. They can be filtered and scatter light.
Colloids: Mixtures with intermediate particle size. Particles do not settle and cannot be filtered out, but they do scatter light (Tyndall effect).
The chapter ends by emphasizing the practical importance of identifying pure substances and mixtures, especially in fields like medicine, food processing, and industry. It encourages students to look critically at substances around them and understand the processes used to purify and separate materials in daily life and laboratories.
Thus, this chapter lays the foundation for understanding the chemical nature of matter and prepares students for advanced topics in chemistry.
————————————————————————————————————————————————————————————————————————–
TEXTBOOK QUESTIONS
🔷 Question 1. Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water
🟢 Evaporation or Crystallisation
(b) Ammonium chloride from a mixture of sodium chloride and ammonium chloride
🟢 Sublimation (since ammonium chloride sublimes on heating)
(c) Small pieces of metal in engine oil of a car
🟢 Filtration or Magnetic separation (if metal is magnetic)
(d) Different pigments from an extract of flower petals
🟢 Chromatography
(e) Butter from curd
🟢 Centrifugation
(f) Oil from water
🟢 Separating funnel
(g) Tea leaves from tea
🟢 Filtration
(h) Iron pins from sand
🟢 Magnetic separation
(i) Wheat grains from husk
🟢 Winnowing
(j) Fine mud particles suspended in water
🟢 Centrifugation or Sedimentation and Decantation
🔷 Question 2. Write the steps you would use for making tea using these words:
solution, solvent, solute, dissolve, soluble, insoluble, filtrate, residue.
🟢 Answer:
Water is taken as the solvent.
Tea leaves, sugar and milk are the solutes.
Sugar and milk dissolve as they are soluble.
Tea leaves are insoluble, so after boiling, they are separated by filtration.
The liquid obtained is the filtrate, and the undissolved tea leaves are the residue.
The final product is a solution (tea).
🔷 Question 3. Pragya tested the solubility of three different substances at different temperatures.
Based on the table:
(a) What mass of potassium nitrate is needed to make a saturated solution in 50 g of water at 313 K?
🟢 Solubility at 313 K = 106 g per 100 g water
→ For 50 g water = (106 × 50) ÷ 100 = 53 g
(b) What will happen if a saturated solution of potassium chloride at 353 K is cooled to room temperature?
🟢 As temperature decreases, solubility decreases.
→ The excess salt will crystallise out.
(c) At 293 K, which salt has the highest solubility?
🟢 Ammonium chloride – 37 g
(d) Effect of temperature on solubility of a salt?
🟢 Generally, solubility increases with increase in temperature.
🔷 Question 4. Explain the following with examples:
(a) Saturated solution
🟢 A solution where no more solute can dissolve at a given temperature.
Example: 36 g NaCl in 100 g water at 293 K
(b) Pure substance
🟢 Substance made of only one kind of particle.
Example: distilled water, gold
(c) Colloid
🟢 A heterogeneous mixture with particles that scatter light but do not settle.
Example: milk, fog
(d) Suspension
🟢 Heterogeneous mixture with visible particles that settle down.
Example: sand in water
🔷 Question 5. Classify the following as homogeneous or heterogeneous mixtures:
soda water, wood, air, soil, vinegar, filtered tea
🟢 Homogeneous mixtures: soda water, air, vinegar, filtered tea
🟡 Heterogeneous mixtures: wood, soil
🔷 Question 6. How would you confirm that a colourless liquid is pure water?
🟢 Answer:
Its boiling point should be exactly 100°C (373 K).
It should have a fixed melting point of 0°C (273 K).
It should not conduct electricity (pure water is a poor conductor).
No residue should remain after evaporation.
🔷 Question 7. Which of the following are pure substances?
🟢 Pure substances:
(a) Ice
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
🔴 Not pure substances:
(b) Milk
(g) Brick
(h) Wood
(i) Air
🔷 Question 8. Identify the solutions among the following:
🟢 Solutions:
(b) Sea water
(c) Air
(e) Soda water
🔴 Not solutions:
(a) Soil
(d) Coal
🔷 Question 9. Which of the following shows Tyndall effect?
🟢 Answer:
✅ (b) Milk
✅ (d) Starch solution
❌ Salt and copper sulphate solutions do not show Tyndall effect as they are true solutions.
🔷 Question 10. Classify the following into elements, compounds and mixtures:
✅ Elements: Sodium, Silver, Tin, Silicon, Mercury
✅ Compounds: Calcium carbonate, Methane, Carbon dioxide
✅ Mixtures: Soil, Sugar solution, Coal, Air, Soap, Blood
🔷 Question 11. Which of the following are chemical changes?
🟢 Chemical changes:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion of food
(g) Burning of a candle
🔴 Not chemical changes:
(c) Mixing iron filings and sand
(f) Freezing of water
————————————————————————————————————————————————————————————————————————–
OTHER IMPORTANT QUESTIONS FOR EXAMS
(MCQs)
1. Which of the following is a pure substance?
A) Milk
B) Air
C) Distilled water
D) Salt solution
Answer: C) Distilled water
2. A mixture of salt and water can be separated by:
A) Filtration
B) Decantation
C) Evaporation
D) Sublimation
Answer: C) Evaporation
3. Which of the following is a heterogeneous mixture?
A) Sugar solution
B) Air
C) Soil
D) Vinegar
Answer: C) Soil
4. The method used to separate cream from milk is:
A) Filtration
B) Centrifugation
C) Evaporation
D) Distillation
Answer: B) Centrifugation
5. The Tyndall effect is shown by:
A) True solutions
B) Suspensions only
C) Colloids only
D) Colloids and suspensions
Answer: D) Colloids and suspensions
Very Short Answer Questions (1–2 lines)
1. Define a pure substance.
Answer: A pure substance is made up of only one kind of particle and has a fixed composition.
2. Name a technique used to separate dyes in ink.
Answer: Chromatography.
3. What is a solute?
Answer: The substance that dissolves in a solvent to form a solution.
4. Which process is used to obtain fresh water from seawater?
Answer: Distillation.
5. Name one substance that shows sublimation.
Answer: Camphor.
Short Answer Questions (30–50 words)
1. Differentiate between a compound and a mixture.
Answer: A compound is formed by chemically combining elements in a fixed ratio and has new properties, while a mixture is formed by physically combining substances in any ratio and retains the properties of its components.
2. What is the Tyndall effect? In which type of mixture is it observed?
Answer: Tyndall effect is the scattering of light by particles in a mixture. It is observed in colloidal and some suspension mixtures, not in true solutions.
3. State any two differences between homogeneous and heterogeneous mixtures.
Answer:
Homogeneous mixtures have a uniform composition; heterogeneous mixtures do not.
Homogeneous mixtures have no visible boundaries; heterogeneous mixtures show visible boundaries between components.
4. What is centrifugation? Mention one of its applications.
Answer: Centrifugation is a method of separating suspended particles from a liquid by spinning it rapidly. It is used to separate cream from milk.
5. How would you separate a mixture of ammonium chloride and sand? Explain.
Answer: The mixture can be separated by sublimation. Ammonium chloride sublimes directly into gas on heating, leaving sand behind. The vapors are then condensed back into solid ammonium chloride.
————————————————————————————————————————————————————————————————————————–
MNEMONICS
————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN

————————————————————————————————————————————————————————————————————————————