Class : 9 – Science (English) : Lesson 1. Matter in Our Surroundings
EXPLANATION & SUMMARY
🟢 Early Classifications of Matter
In ancient times, Indian philosophers classified matter into five basic elements (Panch Tatva):
Air (Vayu)
Water (Jal)
Earth (Prithvi)
Fire (Agni)
Sky/Space (Aakash)
Modern science classifies matter based on two physical properties:
✔ Physical Nature
✔ Chemical Nature
In this chapter, we focus on physical properties of matter.
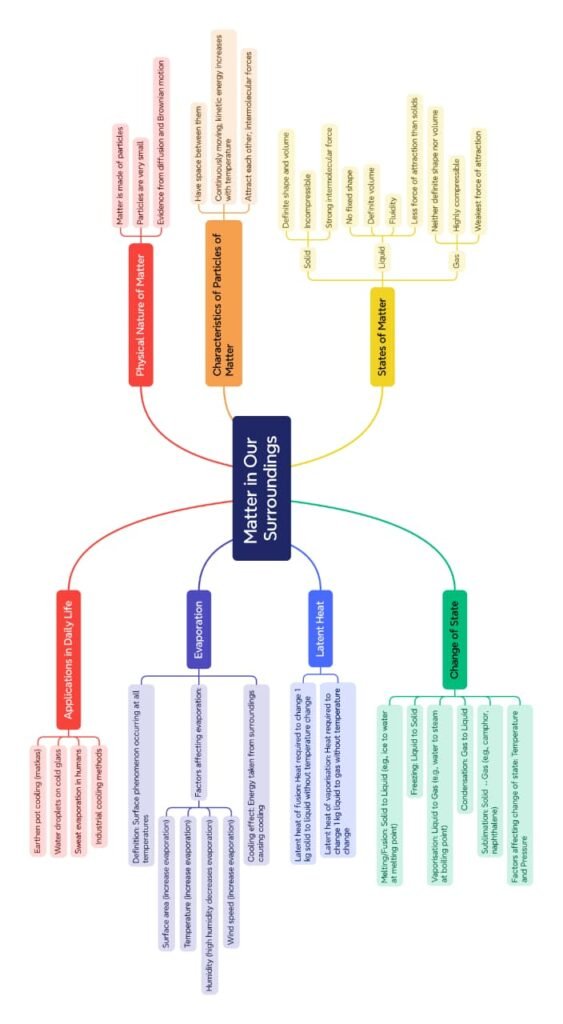
🔴 Physical Nature of Matter
➡ 1. Matter is made up of particles
Scientific experiments, such as dissolving sugar in water, show that matter is made of tiny particles that are too small to be seen with the naked eye.
➡ 2. Particles of matter are very small
Even a single drop of ink can spread in water and color the whole solution. This proves that the particles are microscopic and numerous.
➡ 3. Particles of matter have space between them
When you mix salt in water, it disappears. This shows that the salt particles occupy the spaces between water particles.
➡ 4. Particles of matter are continuously moving
The particles of matter are in constant motion. This motion is called kinetic energy. As the temperature increases, the movement becomes faster.
➡ 5. Particles of matter attract each other
There is a force of attraction between particles, which keeps them bound together. This force is strongest in solids and weakest in gases.
🟡 States of Matter
Matter exists in three primary states:
🔷 1. Solid
🔹 Particles are tightly packed
🔹 Definite shape and volume
🔹 Incompressible and rigid
🔹 Examples: Stone, iron, ice

🔷 2. Liquid
🔹 Particles are loosely packed
🔹 No fixed shape, but fixed volume
🔹 Flows easily
🔹 Examples: Water, oil, milk
🔷 3. Gas
🔹 Particles are far apart and move freely
🔹 No fixed shape or volume
🔹 Highly compressible
🔹 Examples: Air, hydrogen, oxygen
💡 Concept: The arrangement of particles and the strength of intermolecular forces decide the state of matter.
🔵 Can Matter Change Its State?
Yes! Matter can change from one state to another when temperature or pressure changes.
There are interconversions of states of matter:
🧊 1. Solid to Liquid (Melting)
When a solid is heated, its particles gain energy and start vibrating faster. At a certain temperature (called the melting point), it changes to liquid.
🌡 Melting Point of ice = 0°C
💨 2. Liquid to Gas (Boiling or Vaporisation)
Heating a liquid increases the kinetic energy of particles. At a specific temperature (called the boiling point), the liquid changes to gas.
🌡 Boiling Point of water = 100°C
🧊 3. Gas to Liquid (Condensation)
Cooling a gas removes energy, and the particles come closer to form a liquid.
🧊 4. Liquid to Solid (Freezing)
Further cooling leads to freezing. Water changes to ice at 0°C.
🌫 5. Solid to Gas (Sublimation)
Some solids like camphor or naphthalene directly change into gas without becoming liquid. This process is called sublimation.

🧠 Tip: Reversible changes! These changes can be reversed by reversing the temperature or pressure.
🟢 Effect of Change of Temperature
Heat increases the kinetic energy of particles, making them move faster and overcome the forces of attraction between them.
➡ Solids melt into liquids
➡ Liquids boil into gases
On cooling, the reverse happens.
🔴 Effect of Change of Pressure
When we increase pressure, gases can be compressed to form liquids. This is the principle behind liquefied petroleum gas (LPG).
📦 Gases are stored in cylinders under high pressure.
🔵 Latent Heat: Hidden Energy
Latent heat is the hidden energy absorbed or released during a change of state, without changing the temperature.
✔ Latent Heat of Fusion
Heat required to change 1 kg of solid into liquid at its melting point without temperature change.
✔ Latent Heat of Vaporisation
Heat required to change 1 kg of liquid into gas at its boiling point without temperature change.
💡 Why sweat cools us: The latent heat of vaporisation absorbs body heat during evaporation of sweat.
🟡 Evaporation: Cooling Without Boiling
Evaporation is the process where a liquid changes into vapour below its boiling point. It happens at any temperature.
✅ Factors affecting evaporation:
🔥 Temperature: Higher temperature increases evaporation.
🌬 Wind speed: More wind, more evaporation.
📏 Surface area: Larger surface = faster evaporation.
💧 Humidity: Less humidity = more evaporation.
🌿 Real-life examples:
✔ Wet clothes dry faster in sun and wind
✔ Water kept in earthen pots stays cool
✔ Desert coolers work better in dry weather
🟢 Differences Between Boiling and Evaporation
Feature Boiling Evaporation
Temperature At boiling point only At any temperature
Heat Source Requires heat source Uses ambient heat
Speed Fast Slow
Whole/Limited Whole liquid boils Only surface evaporates
✏ Note: Evaporation causes cooling because it takes away heat from the surface.
🔴 Plasma and Bose-Einstein Condensate
In addition to solid, liquid, and gas, there are two more states of matter:
🌀 Plasma
✔ Found in stars and neon signs
✔ Ionized gas with free-moving electrons and ions
✔ Highly energetic and conducts electricity
❄ Bose-Einstein Condensate (BEC)
✔ Discovered by Satyendra Nath Bose and Albert Einstein
✔ Occurs at near absolute zero temperature
✔ Atoms lose energy and form a single quantum entity
🌟 Conclusion
Matter surrounds us in many forms – solid, liquid, gas, plasma, and BEC. Changes in temperature and pressure can shift matter from one state to another. Understanding these basic concepts helps us relate science to real life – from cooking to cooling, from LPG cylinders to air conditioners.
📌 Summary (300 Words)
🔹 Matter is anything that has mass and occupies space. It is made of particles that are very small, have spaces between them, constantly move, and attract each other.
🔹 There are three primary states of matter – solid, liquid, and gas. Solids have fixed shape and volume, liquids have fixed volume but no shape, and gases have neither.
🔹 Matter changes state when temperature or pressure is changed.
Melting: Solid → Liquid
Boiling: Liquid → Gas
Condensation: Gas → Liquid
Freezing: Liquid → Solid
Sublimation: Solid → Gas directly
🔹 The latent heat of fusion and latent heat of vaporisation are the heat energies required to change the state of matter without a change in temperature.
🔹 Evaporation is the slow process of changing a liquid into gas at room temperature. It depends on surface area, temperature, wind speed, and humidity. Evaporation causes cooling.
🔹 Boiling happens at a fixed temperature (boiling point), while evaporation occurs at all temperatures.
🔹 Two additional states of matter include:
Plasma: Ionized gas found in stars and neon lights
Bose-Einstein Condensate (BEC): Supercooled matter found at near absolute zero
🔹 Real-life applications of these concepts include:
Sweating cools the body
LPG stored by compressing gas
Clothes dry faster on windy, hot days
Use of dry ice and camphor shows sublimation
🧠 Conceptual clarity of matter’s states and changes is the foundation for advanced topics in chemistry and physics
————————————————————————————————————————————————————————————————————————–
TEXTBOOK QUESTIONS
Q1. Convert the following temperatures to the Celsius scale:
a. 293 K
b. 470 K
Answer:
To convert a temperature from Kelvin to Celsius, subtract 273 from the Kelvin value.
For 293 K: 293 K − 273 = 20 °C
For 470 K: 470 K − 273 = 197 °C
This conversion is essential when comparing thermal measurements in everyday contexts, since Celsius is the common human-scale unit for temperature.
Question 2
Convert the following temperatures to the Kelvin scale:
a. 25 °C
b. 373 °C
Answer:
To convert from Celsius to Kelvin, add 273 to the Celsius temperature.
For 25 °C: 25 °C + 273 = 298 K
For 373 °C: 373 °C + 273 = 646 K
Using the Kelvin scale is crucial in scientific calculations where absolute temperature is required.
Question 3
Give reasons for the following observations:
a. Naphthalene balls disappear with time without leaving any solid.
b. We can get the smell of perfume sitting several metres away.
Answer:
a. Naphthalene undergoes sublimation—change directly from solid to gas—at room temperature. Its molecules escape into the air, so the balls vanish without melting into a liquid.
b. Perfume consists of volatile liquid molecules that evaporate into the air. Their rapid diffusion through the gas medium carries the fragrance over large distances.
Both phenomena illustrate that certain substances can change state or diffuse at room conditions due to the kinetic energy and spacing between particles.
Question 4
Arrange the following substances in increasing order of forces of attraction between the particles: water, sugar, oxygen.
Answer:
The strength of intermolecular forces increases from gas to solid:
Oxygen (gas)—weak van der Waals forces
Water (liquid)—strong hydrogen bonding
Sugar (solid crystalline)—strong ionic or hydrogen networks
Thus, the order is: Oxygen < Water < Sugar. Weaker forces in gases allow high particle mobility, whereas solids exhibit rigid structures.
Question 5
What is the physical state of water at:
a. 25 °C
b. 0 °C
c. 100 °C
Answer:
a. At 25 °C, water is in the liquid state because it lies between its freezing point (0 °C) and boiling point (100 °C).
b. At 0 °C, water and ice coexist in equilibrium; it is both solid and liquid at the melting/freezing point.
c. At 100 °C, water begins to boil at atmospheric pressure and changes into vapour (gas).
These transitions reflect the effect of temperature on the state of matter.
Question 6
Give two reasons to justify:
a. Water at room temperature is a liquid.
b. An iron almirah is a solid at room temperature.
Answer:
a. Water remains liquid at room temperature because its intermolecular hydrogen bonds are partially overcome, allowing fluid flow but not enough to vaporize. It has enough kinetic energy to move yet remains bound in liquid form.
b. Iron almirah is solid since metallic bonds in iron hold atoms in fixed positions, giving it a definite shape and volume and resisting flow at ambient conditions.
State persistence depends on the balance between kinetic energy and intermolecular forces.
Question 7
Why is ice at 273 K more effective in cooling than water at the same temperature?
Answer:
Ice absorbs the latent heat of fusion when melting, taking up extra energy from its surroundings without a temperature rise. As ice converts to water at 273 K, it removes more heat—cooling surroundings more effectively than liquid water at the same temperature.
Question 8
What produces more severe burns, boiling water or steam?
Answer:
Steam causes more severe burns because, upon condensing on skin, it releases both its sensible heat at 100 °C and the latent heat of vaporisation, delivering a greater total energy release than boiling water alone. This extra heat transfer intensifies the burn.
Question 9
Name A, B, C, D, E and F in the following state-change diagram:↑ B ↑ Gas ← + Liquid A → C ↓ ↓ D ↓ Solid ← + ↑ E ↓
Answer:
A: Sublimation (solid → gas)
B: Vaporisation (liquid → gas)
C: Condensation (gas → liquid)
D: Freezing or Solidification (liquid → solid)
E: Melting or Fusion (solid → liquid)
F: Deposition (gas → solid)
These six processes illustrate all possible interconversions among the three states of matter.
————————————————————————————————————————————————————————————————————————-
OTHER IMPORTANT QUESTIONS FOR EXAMS
🔹 (MCQs) – (5 Questions)
1.Which of the following is not a characteristic of particles of matter?
A. They have spaces between them
B. They are at rest
C. They attract each other
D. They are constantly moving
Answer: B. They are at rest
2.Which of the following is the best evidence for the motion of particles in matter?
A. Evaporation
B. Diffusion
C. Melting
D. Condensation
Answer: B. Diffusion
3.Which state of matter has a definite volume but no definite shape?
A. Solid
B. Liquid
C. Gas
D. Plasma
Answer: B. Liquid
4.Which of these processes involves change of state from gas to liquid?
A. Sublimation
B. Evaporation
C. Condensation
D. Melting
Answer: C. Condensation
5.The boiling point of water is affected by:
A. Temperature only
B. Pressure only
C. Both temperature and pressure
D. Neither temperature nor pressure
Answer: C. Both temperature and pressure
🔹 Fill in the Blanks – (3 Questions)
6. The phenomenon of change of a solid directly into gas is called __.
Answer: Sublimation
Particles of matter are in continuous __.
Answer: Motion
7. The temperature at which a liquid starts boiling at atmospheric pressure is called its __.
Answer: Boiling point
🔹 True or False – (2 Questions)
8. In gases, the intermolecular forces are stronger than in solids.
Answer: False
The smell of perfume spreads due to diffusion.
Answer: True
🔹 Short Answer Questions – (5 Questions)
9. What are the characteristics of particles of matter?
Answer:
Particles of matter have the following characteristics:
They are very small.
They have spaces between them.
They are in constant motion.
They attract each other.
10.Define latent heat of vaporization.
Answer:
Latent heat of vaporization is the amount of heat required to convert 1 kg of a liquid into vapor at its boiling point without any change in temperature.
11. Why do gases exert pressure on the walls of the container?
Answer:
Gases exert pressure on the walls of the container because their particles move randomly and collide with the walls, exerting force per unit area.
1
2. How is the compressibility of gases different from solids and liquids?
Answer:
Gases are highly compressible due to the large spaces between particles, unlike solids and liquids which have tightly packed particles and are hardly compressible.
13. Explain why water kept in an earthen pot becomes cool during summer.
Answer:
Water in an earthen pot becomes cool because the porous walls allow water to seep out and evaporate. Evaporation requires latent heat, which is taken from the remaining water, lowering its temperature.
————————————————————————————————————————————————————————————————————————-
MNEMONICS
————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN

————————————————————————————————————————————————————————————————————————————