Class 12 : Physics (English) – Chapter 11: Dual Nature of Radiation and Matter
EXPLANATION & SUMMARY
🌟 Introduction
The concept of the dual nature of radiation and matter revolutionised modern physics. Initially, light was treated purely as a wave, supported by experiments of interference, diffraction, and polarisation. However, phenomena like the photoelectric effect and black-body radiation could not be explained by wave theory.
Albert Einstein’s photon theory of light provided the breakthrough, introducing the concept of light quanta. Later, Louis de Broglie extended this duality to matter particles, predicting that they too possess wave-like properties. The famous Davisson–Germer experiment confirmed this.
This chapter unifies the wave and particle aspects of both radiation and matter, laying the foundation of quantum mechanics.
🔵 1. Photoelectric Effect – Experimental Observations

Discovered by Hertz (1887), studied by Lenard.
When light of sufficiently high frequency shines on a metallic surface, electrons are emitted → called photoelectrons.
✔️ Observations:
Threshold frequency (ν₀): Below a certain frequency, no photoemission occurs, no matter how intense the light.
Instantaneous emission: Photoelectrons are emitted immediately, even under weak light.
Kinetic energy ∝ frequency: KE of emitted electrons increases with frequency, not with intensity.
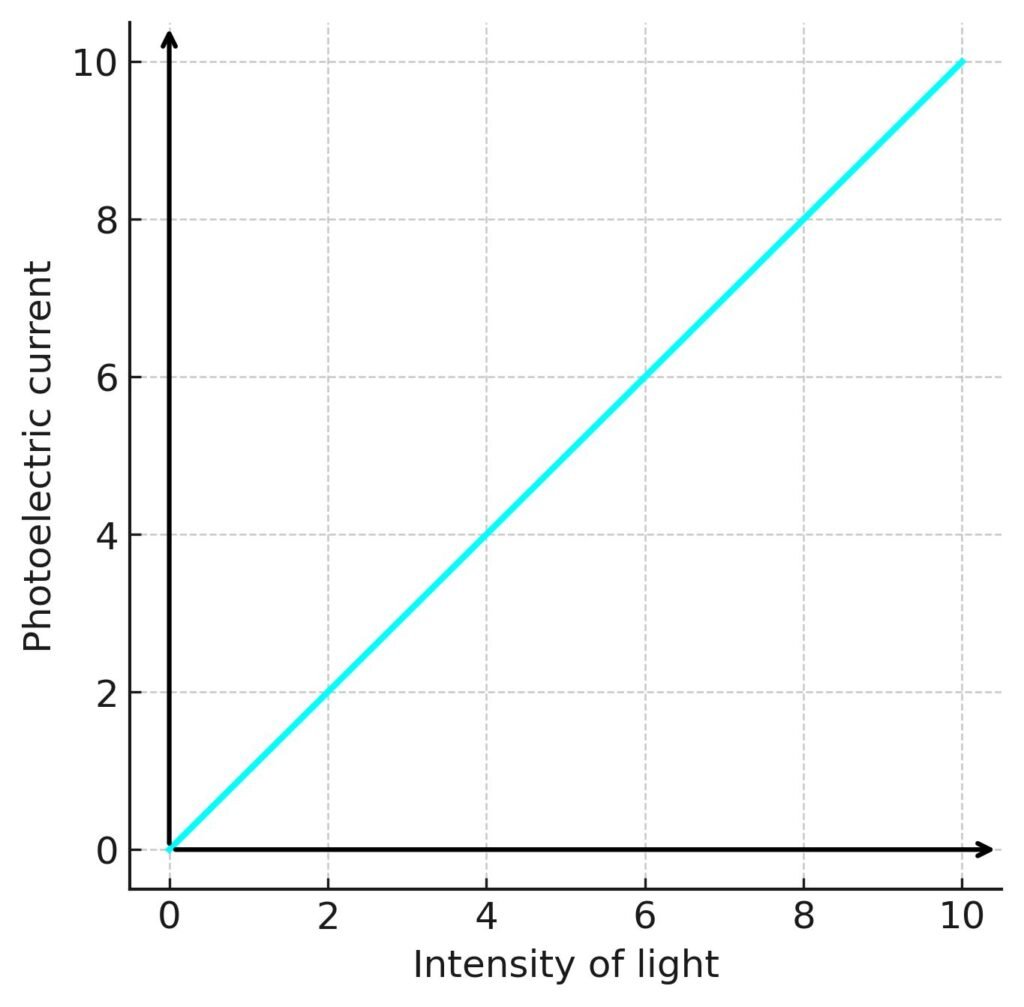
Current ∝ intensity: Number of photoelectrons depends on intensity.

💡 Wave theory failed: It predicted energy ∝ intensity, but experiments showed energy depends on frequency.
🟢 2. Einstein’s Quantum Explanation
Einstein (1905) applied Planck’s quantum theory to explain photoelectric emission.
Light consists of photons (energy packets).
Photon energy: E = hν.
One photon → interacts with one electron.
If photon energy ≥ work function (φ), emission occurs.
⚡ Einstein’s Photoelectric Equation:
hν = φ + Kmax
➡️ Kmax = hν – φ
Also,
Kmax = eVs → eVs = hν – φ
🔴 3. Millikan’s Experimental Verification
Although Einstein proposed this in 1905, his theory was confirmed much later. In 1916–1919, Robert Millikan performed detailed experiments.
He measured stopping potential (Vs) for different frequencies.
Plotted Vs vs ν graph → obtained a straight line.
Slope = h/e → verified Planck’s constant.
Extrapolation gave threshold frequency (ν₀).
✔️ Millikan experimentally proved the validity of Einstein’s photoelectric equation.
🟡 4. Work Function (φ) and Threshold Frequency (ν₀)
Work Function (φ): Minimum energy needed to eject an electron.
Depends on material (1–5 eV typical).
Threshold Frequency (ν₀): Minimum light frequency required.
Relation: φ = hν₀
🔵 5. Wave–Particle Duality of Light
Wave nature: interference, diffraction, polarisation.
Particle nature: photoelectric effect, Compton effect.
Hence, light has dual character.
🟢 6. de Broglie Hypothesis (Matter Waves)
Proposed by Louis de Broglie (1924): particles like electrons have wavelength.
λ = h/p = h/(mv)
For an electron accelerated through potential V:
λ = h / √(2meV)
💡 Very small λ → wave nature only observable in microscopic particles.
🔴 7. Davisson–Germer Experiment
1927 → Confirmed de Broglie waves.
Electrons fired at nickel crystal.
Detected diffraction maxima at certain angles.
Observed wavelength matched de Broglie prediction.
✔️ Proved wave nature of electrons.
🟡 8. Applications of Dual Nature
Photoelectric cells: light sensors, automatic doors, TV camera tubes.
Electron microscope: uses electron beams with small λ → higher resolving power.
X-ray diffraction & crystallography: structural studies.
Quantum mechanics foundation: explains atomic structure, semiconductors, nanotechnology.
🔵 9. Limitations & Significance
For macroscopic objects, de Broglie λ negligible → wave effects unobservable.
For microscopic particles, λ significant → diffraction/interference possible.
Duality is universal and forms basis of modern quantum physics.
🔴 10. Numerical Illustrations
Example 1:
Threshold frequency ν₀ = 8×10¹⁴ Hz. Find stopping potential if light of ν = 1.2×10¹⁵ Hz falls on it.
h = 6.63×10⁻³⁴ J·s, e = 1.6×10⁻¹⁹ C
E = hν = 7.96×10⁻¹⁹ J = 4.97 eV
φ = hν₀ = 3.31 eV
Kmax = E – φ = 1.66 eV
Vs = Kmax/e = 1.66 V
✔️ Stopping potential = 1.66 V
Example 2:
Electron accelerated through 100 V. Find λ.
λ = h/√(2meV)
= 6.63×10⁻³⁴ / √(2×9.1×10⁻³¹×1.6×10⁻¹⁹×100)
≈ 1.23×10⁻¹⁰ m = 0.123 nm
✔️ λ = 0.123 nm
📘 Summary (~300 Words)
Photoelectric Effect: Emission of electrons when light ≥ threshold frequency falls on metal. Observed by Hertz, studied by Lenard.
Einstein’s Explanation: Light is photon packets (E = hν). Equation: hν = φ + Kmax. Verified by Millikan.
Work Function (φ): Minimum energy to remove electron. Threshold frequency ν₀ = φ/h.
Wave–Particle Duality: Light shows both wave (interference, diffraction) and particle (photoelectric effect) behaviour.
de Broglie Hypothesis: Matter waves with λ = h/p. For electron: λ = h/√(2meV).
Davisson–Germer Experiment: Confirmed wave nature of electrons.
Applications: Photoelectric cells, electron microscopes, crystallography, quantum devices.
Significance: Dual nature is universal, forming the foundation of quantum mechanics.
📝 Quick Recap
✔️ Photoelectric effect proved particle nature of light.
✔️ Einstein’s equation: eVs = hν – φ.
✔️ Millikan verified Einstein’s theory.
✔️ de Broglie: λ = h/p → matter waves.
✔️ Davisson–Germer confirmed electron diffraction.
✔️ Applications: photoelectric devices, electron microscope, quantum physics foundation.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
🔷 Q11.1
Find the
(a) maximum frequency, and
(b) minimum wavelength of X-rays produced by 30 kV electrons.
Answer
⚡ Energy of electron = eV = (1.6×10^-19)(3.0×10^4) = 4.81×10^-15 J
(a) f_max = E/h = (4.81×10^-15)/(6.63×10^-34) ≈ 7.25×10^18 Hz
(b) λ_min = hc/E = (6.63×10^-34×3×10^8)/(4.81×10^-15) ≈ 4.13×10^-11 m (0.041 nm)
🔷 Q11.2
The work function of caesium metal is 2.14 eV. When light of frequency 6×10^14 Hz is incident on the metal surface, photoemission of electrons occurs. What is the
(a) maximum kinetic energy of the emitted electrons,
(b) Stopping potential, and
(c) maximum speed of the emitted photoelectrons?
Answer
Photon energy = hf = (6.63×10^-34)(6×10^14) = 3.98×10^-19 J = 2.48 eV
(a) KE_max = 2.48 − 2.14 = 0.34 eV = 5.5×10^-20 J
(b) Stopping potential = 0.34 V
(c) v_max = √(2KE/m) = √(2×5.5×10^-20 / 9.11×10^-31) ≈ 3.5×10^5 m/s
🔷 Q11.3
The photoelectric cut-off voltage in a certain experiment is 1.5 V. What is the maximum kinetic energy of photoelectrons emitted?
Answer
KE_max = eV = (1.6×10^-19)(1.5) = 2.4×10^-19 J = 1.5 eV
🔷 Q11.4
Monochromatic light of wavelength 632.8 nm is produced by a helium-neon laser. The power emitted is 9.42 mW.
(a) Find the energy and momentum of each photon in the light beam,
(b) How many photons per second, on the average, arrive at a target irradiated by this beam? (Assume the beam to have uniform cross-section which is less than the target area), and
(c) How fast does a hydrogen atom have to travel, in order to have the same momentum as that of the photon?
Answer
(a) E = hc/λ = (6.63×10^-34×3×10^8)/(6.33×10^-7) ≈ 3.14×10^-19 J (1.96 eV)
p = h/λ ≈ 1.05×10^-27 kg·m/s
(b) N/s = P/E = (9.42×10^-3)/(3.14×10^-19) ≈ 3.0×10^16 photons/s
(c) v = p/m_H = (1.05×10^-27)/(1.67×10^-27) ≈ 0.63 m/s
🔷 Q11.5
In an experiment on photoelectric effect, the slope of the cut-off voltage versus frequency of incident light is found to be 4.12×10^-15 V·s. Calculate the value of Planck’s constant.
Answer
h = slope×e = (4.12×10^-15)(1.6×10^-19) = 6.6×10^-34 J·s
🔷 Q11.6
The threshold frequency for a certain metal is 3.3×10^14 Hz. If light of frequency 8.2×10^14 Hz is incident on the metal, predict the cut-off voltage for the photoelectric emission.
Answer
Δf = 8.2 − 3.3 = 4.9×10^14 Hz
V₀ = hΔf/e = (6.63×10^-34×4.9×10^14)/(1.6×10^-19) ≈ 2.0 V
🔷 Q11.7
The work function for a certain metal is 4.2 eV. Will this metal give photoelectric emission for incident radiation of wavelength 330 nm?
Answer
E = 1240/330 ≈ 3.76 eV
Since E < ϕ, ❌ no emission
🔷 Q11.8
Light of frequency 7.21×10^14 Hz is incident on a metal surface. Electrons with a maximum speed of 6.0×10^5 m/s are ejected from the surface. What is the threshold frequency for photoemission of electrons?
Answer
KE = ½mv² = 0.5×9.11×10^-31×(6×10^5)^2 ≈ 1.64×10^-19 J
f₀ = f − KE/h = 7.21×10^14 − (1.64×10^-19 / 6.63×10^-34)
f₀ ≈ 4.74×10^14 Hz
🔷 Q11.9
Light of wavelength 488 nm is produced by an argon laser which is used in the photoelectric effect. When light from this spectral line is incident on the emitter, the stopping (cut-off) potential of photoelectrons is 0.38 V. Find the work function of the material from which the emitter is made.
Answer
E = hc/λ = (6.63×10^-34×3×10^8)/(4.88×10^-7) ≈ 2.54 eV
KE = eV₀ = 0.38 eV
ϕ = E − KE = 2.54 − 0.38 = 2.16 eV
🔷 Q11.10
What is the de Broglie wavelength of
(a) a bullet of mass 0.040 kg travelling at the speed of 1.0 km/s,
(b) a ball of mass 0.060 kg moving at a speed of 1.0 m/s, and
(c) a dust particle of mass 1.0×10^-9 kg drifting with a speed of 2.2 m/s?
Answer
(a) λ = h/mv = 6.63×10^-34 / (0.040×1000) ≈ 1.7×10^-35 m
(b) λ = 6.63×10^-34 / (0.060×1.0) ≈ 1.1×10^-32 m
(c) λ = 6.63×10^-34 / (1.0×10^-9×2.2) ≈ 3.0×10^-25 m
🔷 Q11.11
Show that the wavelength of electromagnetic radiation is equal to the de Broglie wavelength of its quantum (photon).
Answer
For photon: E = hf = pc → p = h/λ
De Broglie λ = h/p = h/(h/λ) = λ
✔️ Hence proved.
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS
(CBSE MODEL QUESTION PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
✨ Section A (Q1–Q18: 1 Mark Each)
🔹 Q1. Who first discovered the photoelectric effect?
Answer: Heinrich Hertz in 1887.
🔹 Q2. Write Einstein’s photoelectric equation.
Answer:
hf = ϕ + KE_max
where hf = photon energy, ϕ = work function, KE_max = maximum kinetic energy of electron.
🔹 Q3. Define work function of a metal.
Answer: Minimum energy required to just eject an electron from a metal surface.
🔹 Q4. State one difference between emission of light in photoelectric effect and emission due to heating of metal.
Answer:
Photoelectric emission occurs instantly without delay,
Thermionic emission requires heating and time lag.
🔹 Q5. Write the expression for de Broglie wavelength of an electron accelerated through potential V.
Answer:
λ = h / √(2meV)
🔹 Q6. Which experiment confirmed the wave nature of electrons?
Answer: Davisson and Germer experiment.
🔹 Q7. Give one application of photoelectric effect.
Answer: Photoelectric cells used in automatic streetlights.
🔹 Q8. State the unit of Planck’s constant.
Answer: Joule second (J·s).
🔹 Q9. Define threshold frequency.
Answer: The minimum frequency of incident radiation required to eject electrons from the metal surface.
🔹 Q10. What is the slope of V–f graph in photoelectric effect?
Answer: h/e (Planck’s constant per unit charge).
🔹 Q11. Calculate photon energy in eV for light of wavelength 620 nm.
Answer:
E = 1240/λ = 1240/620 ≈ 2.0 eV
🔹 Q12. Mention one property of photoelectric emission not explained by wave theory of light.
Answer: Instantaneous emission without time lag.
🔹 Q13. What happens to stopping potential if intensity of incident light is doubled?
Answer: It remains unchanged.
🔹 Q14. Write de Broglie relation for wavelength of matter waves.
Answer: λ = h/p
🔹 Q15. A photon and an electron have same de Broglie wavelength. Which has higher momentum?
Answer: Both have equal momentum since λ = h/p.
🔹 Q16. If the frequency of incident light is below threshold, what will happen?
Answer: No photoelectrons are emitted, irrespective of intensity.
🔹 Q17. Name the scientist who explained photoelectric effect using quantum theory of light.
Answer: Albert Einstein (1905).
🔹 Q18. State the nature of relationship between KE_max and frequency of incident light.
Answer: Linear relation: KE_max = hf − ϕ.
✨ Section B (Q19–Q23: 2 Marks Each)
🔹 Q19. A photon has energy of 2 eV. Calculate its wavelength.
Answer:
λ = 1240/E = 1240/2 = 620 nm
🔹 Q20. What is the stopping potential required to stop emission of electrons having KE_max = 2 eV?
Answer:
V = KE/e = 2/1 = 2 V
🔹 Q21. Write two differences between wave nature and particle nature of radiation.
Answer:
Wave nature → diffraction, interference
Particle nature → photoelectric effect, Compton effect
🔹 Q22. Define photon momentum. Derive its expression.
Answer:
p = E/c = hf/c = h/λ.
🔹 Q23. Calculate frequency of light for which photon energy is 3 eV.
Answer:
f = E/h = (3×1.6×10^-19)/(6.63×10^-34) ≈ 7.2×10^14 Hz
✨ Section C (Q24–Q28: 3 Marks Each)
🔹 Q24. State the laws of photoelectric emission.
Answer:
Photoelectric current ∝ intensity of light.
KE_max depends only on frequency, not on intensity.
Emission is instantaneous.
A threshold frequency exists.
🔹 Q25. Light of λ = 400 nm falls on a metal with ϕ = 2 eV. Find KE_max.
Answer:
E = 1240/400 = 3.1 eV
KE_max = 3.1 − 2 = 1.1 eV
🔹 Q26. Why cannot wave theory explain photoelectric effect?
Answer:
Because it fails to explain
Instantaneous emission
Dependence on frequency, not intensity
Existence of threshold frequency
🔹 Q27. Derive relation between stopping potential and frequency of incident radiation.
Answer:
From Einstein: KE_max = hf − ϕ
Also KE_max = eV₀
∴ eV₀ = hf − ϕ
This is the required relation.
🔹 Q28. Calculate wavelength of electron accelerated by potential of 150 V.
Answer:
λ = h/√(2meV)
= 1.227/√V nm
= 1.227/√150 ≈ 0.1 nm
✨ Section D (Q29–Q31: 4 Marks Each)
🔹 Q29. Explain Davisson and Germer experiment to confirm wave nature of electrons.
Answer:
Electrons accelerated by potential ~54 V were made to fall on nickel crystal.
Diffraction pattern similar to X-rays was observed.
Verified de Broglie relation λ = h/p.
✔️ Thus, wave nature of electrons confirmed.
🔹 Q30. A light source of 10 mW emits monochromatic light of λ = 500 nm. How many photons are emitted per second?
Answer:
E = hc/λ = (6.63×10^-34×3×10^8)/(5×10^-7) ≈ 3.98×10^-19 J
N/s = P/E = (10×10^-3)/(3.98×10^-19) ≈ 2.5×10^16 photons/s
🔹 Q31. State experimental observations of photoelectric effect which cannot be explained by classical wave theory.
Answer:
Instantaneous emission of electrons
Existence of threshold frequency
KE_max depends on frequency, not intensity
Current ∝ intensity
✨ Section E (Q32–Q35: 5 Marks Each)
🔹 Q32. Derive Einstein’s photoelectric equation on the basis of quantum theory.
Answer:
A photon of energy hf strikes the electron.
Part of energy is used to overcome work function (ϕ).
Remaining energy appears as KE_max.
∴ hf = ϕ + KE_max
✔️ This is Einstein’s equation.
🔹 Q33. Derive expression for de Broglie wavelength of electron accelerated by potential difference V.
Answer:
Kinetic energy = eV = ½mv²
Momentum p = √(2meV)
λ = h/p = h/√(2meV)
Final: λ (in nm) = 1.227/√V.
🔹 Q34. A metal with work function 2.5 eV is illuminated by radiation of λ = 400 nm. Calculate KE_max, stopping potential and threshold frequency.
Answer:
E = 1240/400 = 3.1 eV
KE = 3.1 − 2.5 = 0.6 eV
Stopping potential = 0.6 V
Threshold frequency f₀ = ϕ/h = (2.5×1.6×10^-19)/(6.63×10^-34) ≈ 6.0×10^14 Hz
🔹 Q35. Explain graph of KE_max versus frequency of incident light in photoelectric effect.
Answer:
Graph is a straight line.
Slope = h (Planck’s constant).
X-intercept = threshold frequency f₀.
Y-intercept = −ϕ (work function).
✔️ It experimentally verifies Einstein’s photoelectric equation.
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
Question 1: The stopping potential for photoelectrons depends on
🔵 (A) frequency of incident light
🟢 (B) intensity of incident light
🟠 (C) wavelength of metal surface
🔴 (D) angle of incidence
Answer: (A) frequency of incident light
Year: 2025
Question 2: Which effect cannot be explained on the basis of wave theory of light?
🔵 (A) Interference
🟢 (B) Diffraction
🟠 (C) Polarisation
🔴 (D) Photoelectric effect
Answer: (D) Photoelectric effect
Year: 2025
Question 3: In a photoelectric experiment, if frequency of incident light is doubled, the maximum kinetic energy of emitted electrons will
🔵 (A) become double
🟢 (B) become more than double
🟠 (C) increase linearly with frequency
🔴 (D) remain same
Answer: (C) increase linearly with frequency
Year: 2025
Question 4: Threshold frequency for a metal surface is 4 × 10¹⁴ Hz. Work function of metal is
🔵 (A) 2.64 eV
🟢 (B) 1.65 eV
🟠 (C) 0.82 eV
🔴 (D) 3.31 eV
Answer: (A) 2.64 eV
Year: 2024
Question 5: In photoelectric effect, if intensity of light is doubled, then
🔵 (A) number of photoelectrons doubles
🟢 (B) maximum kinetic energy doubles
🟠 (C) threshold frequency doubles
🔴 (D) stopping potential doubles
Answer: (A) number of photoelectrons doubles
Year: 2024
Question 6: Which particle shows both wave and particle nature?
🔵 (A) Electron
🟢 (B) Proton
🟠 (C) Neutron
🔴 (D) All of these
Answer: (D) All of these
Year: 2024
Question 7: The De Broglie wavelength of electron accelerated through potential V is proportional to
🔵 (A) 1/√V
🟢 (B) √V
🟠 (C) 1/V
🔴 (D) V
Answer: (A) 1/√V
Year: 2024
Question 8: If frequency of incident light is less than threshold frequency, then photoelectric emission
🔵 (A) does not occur
🟢 (B) occurs with reduced energy
🟠 (C) occurs slowly
🔴 (D) occurs at faster rate
Answer: (A) does not occur
Year: 2023
Question 9: The maximum kinetic energy of emitted photoelectrons is given by
🔵 (A) hν
🟢 (B) hν − Φ
🟠 (C) Φ − hν
🔴 (D) eV₀ − Φ
Answer: (B) hν − Φ
Year: 2023
Question 10: The wavelength of photon having energy 1 eV is approximately
🔵 (A) 1240 nm
🟢 (B) 124 nm
🟠 (C) 12.4 μm
🔴 (D) 1.24 nm
Answer: (A) 1240 nm
Year: 2023
Question 11: The work function of a photoemissive material is 2.0 eV. The threshold wavelength is
🔵 (A) 620 nm
🟢 (B) 540 nm
🟠 (C) 310 nm
🔴 (D) 1240 nm
Answer: (A) 620 nm
Year: 2022
Question 12: A photon of frequency 2 × 10¹⁵ Hz strikes a metal surface with work function 2 eV. The maximum KE of emitted electron is
🔵 (A) 6.28 eV
🟢 (B) 5.28 eV
🟠 (C) 3.28 eV
🔴 (D) 2.28 eV
Answer: (B) 5.28 eV
Year: 2022
Question 13: De Broglie wavelength of particle is inversely proportional to
🔵 (A) momentum
🟢 (B) energy
🟠 (C) velocity
🔴 (D) mass
Answer: (A) momentum
Year: 2022
Question 14: Which of the following is evidence of wave nature of electron?
🔵 (A) Photoelectric effect
🟢 (B) Compton effect
🟠 (C) Davisson-Germer experiment
🔴 (D) Pair production
Answer: (C) Davisson-Germer experiment
Year: 2022
Question 15: The stopping potential depends on
🔵 (A) frequency of incident radiation
🟢 (B) intensity of incident radiation
🟠 (C) both
🔴 (D) neither
Answer: (A) frequency of incident radiation
Year: 2021
Question 16: Which of the following has highest penetrating power?
🔵 (A) α-rays
🟢 (B) β-rays
🟠 (C) γ-rays
🔴 (D) X-rays
Answer: (C) γ-rays
Year: 2021
Question 17: Work function of a surface is 2.76 eV. Threshold frequency is
🔵 (A) 6.7 × 10¹⁴ Hz
🟢 (B) 4.2 × 10¹⁴ Hz
🟠 (C) 2.1 × 10¹⁴ Hz
🔴 (D) 3.3 × 10¹⁴ Hz
Answer: (A) 6.7 × 10¹⁴ Hz
Year: 2021
Question 18: Energy of a photon is given by
🔵 (A) hc/λ
🟢 (B) hν
🟠 (C) both (A) and (B)
🔴 (D) h/ν
Answer: (C) both (A) and (B)
Year: 2021
Question 19: The graph between stopping potential and frequency of incident light is
🔵 (A) straight line
🟢 (B) parabola
🟠 (C) hyperbola
🔴 (D) exponential
Answer: (A) straight line
Year: 2020
Question 20: Davisson and Germer experiment is based on
🔵 (A) Photoelectric effect
🟢 (B) Wave nature of electron
🟠 (C) X-ray diffraction
🔴 (D) Particle nature of electron
Answer: (B) Wave nature of electron
Year: 2020
Question 21: The rest mass of photon is
🔵 (A) zero
🟢 (B) hν/c²
🟠 (C) mc²
🔴 (D) finite
Answer: (A) zero
Year: 2020
Question 22: Work function of sodium is 2.3 eV. Threshold wavelength is
🔵 (A) 540 nm
🟢 (B) 620 nm
🟠 (C) 310 nm
🔴 (D) 1240 nm
Answer: (A) 540 nm
Year: 2019
Question 23: In photoelectric effect, the number of photoelectrons emitted depends on
🔵 (A) intensity of incident light
🟢 (B) frequency of incident light
🟠 (C) work function of metal
🔴 (D) threshold frequency
Answer: (A) intensity of incident light
Year: 2019
Question 24: Which radiation has highest energy photons?
🔵 (A) Ultraviolet
🟢 (B) Infrared
🟠 (C) X-rays
🔴 (D) Gamma rays
Answer: (D) Gamma rays
Year: 2019
Question 25: The graph between KE of photoelectrons and frequency of incident radiation is
🔵 (A) straight line with slope h
🟢 (B) straight line with slope e
🟠 (C) parabola
🔴 (D) hyperbola
Answer: (A) straight line with slope h
Year: 2019
Question 26: The photoelectric effect supports which nature of light?
🔵 (A) Wave nature
🟢 (B) Particle nature
🟠 (C) Both wave and particle
🔴 (D) None
Answer: (B) Particle nature
Year: 2018
Question 27: The de Broglie wavelength of an electron accelerated through potential difference V is
🔵 (A) h/√(2meV)
🟢 (B) h/meV
🟠 (C) √(2meV)/h
🔴 (D) hV/me
Answer: (A) h/√(2meV)
Year: 2018
Question 28: The slope of graph between stopping potential and frequency of incident light gives
🔵 (A) Work function
🟢 (B) Planck’s constant
🟠 (C) Threshold frequency
🔴 (D) Electron charge
Answer: (B) Planck’s constant
Year: 2018
Question 29: A photon of 5 eV falls on a metal surface with work function 3 eV. The maximum KE of electron is
🔵 (A) 2 eV
🟢 (B) 3 eV
🟠 (C) 5 eV
🔴 (D) 8 eV
Answer: (A) 2 eV
Year: 2017
Question 30: The number of photoelectrons emitted per second depends on
🔵 (A) intensity of light
🟢 (B) frequency of light
🟠 (C) work function
🔴 (D) stopping potential
Answer: (A) intensity of light
Year: 2017
Question 31: Photoelectric effect cannot be explained by
🔵 (A) particle nature of light
🟢 (B) wave nature of light
🟠 (C) photon theory
🔴 (D) Einstein’s relation
Answer: (B) wave nature of light
Year: 2017
Question 32: In a photoelectric experiment, the photoelectric current increases with
🔵 (A) frequency of incident light
🟢 (B) intensity of incident light
🟠 (C) work function
🔴 (D) threshold frequency
Answer: (B) intensity of incident light
Year: 2016
Question 33: The minimum frequency of radiation below which photoelectric emission is not possible is called
🔵 (A) Threshold frequency
🟢 (B) Cut-off frequency
🟠 (C) Critical frequency
🔴 (D) Both A and B
Answer: (D) Both A and B
Year: 2016
Question 34: The momentum of a photon of wavelength λ is
🔵 (A) h/λ
🟢 (B) hc/λ
🟠 (C) λ/h
🔴 (D) λ/hc
Answer: (A) h/λ
Year: 2016
Question 35: The energy of a photon of wavelength 5000 Å is approximately
🔵 (A) 2.5 eV
🟢 (B) 3.0 eV
🟠 (C) 4.0 eV
🔴 (D) 5.0 eV
Answer: (A) 2.5 eV
Year: 2015
Question 36: Which experiment demonstrates wave nature of electrons?
🔵 (A) Millikan oil drop
🟢 (B) Davisson-Germer
🟠 (C) Rutherford scattering
🔴 (D) Photoelectric effect
Answer: (B) Davisson-Germer
Year: 2015
Question 37: The kinetic energy of photoelectrons depends on
🔵 (A) intensity of incident light
🟢 (B) frequency of incident light
🟠 (C) surface area of emitter
🔴 (D) power of lamp
Answer: (B) frequency of incident light
Year: 2015
Question 38: Which of the following supports quantisation of energy?
🔵 (A) Photoelectric effect
🟢 (B) Compton effect
🟠 (C) Black body radiation
🔴 (D) All of these
Answer: (D) All of these
Year: 2014
Question 39: The electron microscope works on principle of
🔵 (A) particle nature of electron
🟢 (B) wave nature of electron
🟠 (C) uncertainty principle
🔴 (D) conservation of energy
Answer: (B) wave nature of electron
Year: 2014
Question 40: If frequency of incident light equals threshold frequency, photoelectrons are emitted with
🔵 (A) maximum KE
🟢 (B) zero KE
🟠 (C) minimum KE
🔴 (D) infinite KE
Answer: (B) zero KE
Year: 2014
Question 41: In photoelectric effect, the photoelectric current becomes zero when
🔵 (A) frequency decreases
🟢 (B) intensity decreases
🟠 (C) negative potential applied to collector
🔴 (D) frequency is less than threshold
Answer: (C) negative potential applied to collector
Year: 2013
Question 42: The charge of a photon is
🔵 (A) +e
🟢 (B) −e
🟠 (C) zero
🔴 (D) 1 C
Answer: (C) zero
Year: 2013
Question 43: The de Broglie wavelength of electron accelerated through potential V (in volts) is
🔵 (A) 12.27/√V Å
🟢 (B) √V/12.27 Å
🟠 (C) V/12.27 Å
🔴 (D) 12.27V Å
Answer: (A) 12.27/√V Å
Year: 2012
Question 44: The phenomenon of emission of electrons by metals due to heat energy is
🔵 (A) Thermionic emission
🟢 (B) Field emission
🟠 (C) Photoelectric emission
🔴 (D) Secondary emission
Answer: (A) Thermionic emission
Year: 2012
Question 45: A photocell is used to
🔵 (A) convert heat into electricity
🟢 (B) measure frequency of light
🟠 (C) convert light into electricity
🔴 (D) amplify current
Answer: (C) convert light into electricity
Year: 2011
Question 46: Work function is measured in
🔵 (A) Joule
🟢 (B) Electron volt
🟠 (C) Watt
🔴 (D) Volt
Answer: (B) Electron volt
Year: 2010
Question 47: Which of the following is an application of photoelectric effect?
🔵 (A) Solar cells
🟢 (B) Thermionic valve
🟠 (C) Cyclotron
🔴 (D) Transformer
Answer: (A) Solar cells
Year: 2009
Question 48: The relation between maximum KE of photoelectrons and stopping potential is
🔵 (A) KEmax = eV₀
🟢 (B) KEmax = V₀
🟠 (C) KEmax = hν
🔴 (D) KEmax = hc/λ
Answer: (A) KEmax = eV₀
Year: 2008
Question 49: Who gave the quantum theory of photoelectric effect?
🔵 (A) Planck
🟢 (B) Einstein
🟠 (C) Rutherford
🔴 (D) Bohr
Answer: (B) Einstein
Year: 2007
Question 50: Which of the following equations is Einstein’s photoelectric equation?
🔵 (A) hν = Φ + KEmax
🟢 (B) hν = Φ − KEmax
🟠 (C) KEmax = hν
🔴 (D) KEmax = Φ
Answer: (A) hν = Φ + KEmax
Year: 2006
————————————————————————————————————————————————————————————————————————————–
JEE MAINS QUESTIONS FROM THIS LESSON
Question 1: The de Broglie wavelength of an electron accelerated through potential V is
🔵 (A) h/√(2meV)
🟢 (B) h/√(meV)
🟠 (C) h/√(eV)
🔴 (D) h/√(2V)
Answer: (A) h/√(2meV)
Year: 2025 | Shift 1
Question 2: The threshold frequency for a metal is f₀. The maximum kinetic energy of photoelectrons emitted by light of frequency f is
🔵 (A) hf₀
🟢 (B) hf − hf₀
🟠 (C) hf + hf₀
🔴 (D) hf₀ − hf
Answer: (B) hf − hf₀
Year: 2025 | Shift 2
Question 3: In photoelectric effect, stopping potential depends on
🔵 (A) intensity of light
🟢 (B) frequency of light
🟠 (C) number of electrons emitted
🔴 (D) work function
Answer: (B) frequency of light
Year: 2024 | Jan Shift 1
Question 4: The work function of a metal is 2 eV. The threshold wavelength is approximately
🔵 (A) 1240 nm
🟢 (B) 620 nm
🟠 (C) 310 nm
🔴 (D) 155 nm
Answer: (B) 620 nm
Year: 2024 | Apr Shift 1
Question 5: Einstein’s photoelectric equation is
🔵 (A) hf = ϕ + KEmax
🟢 (B) hf = ϕ − KEmax
🟠 (C) hf = KEmax − ϕ
🔴 (D) hf = ϕ
Answer: (A) hf = ϕ + KEmax
Year: 2024 | Jan Shift 2
Question 6: The photoelectric current is proportional to
🔵 (A) frequency of incident light
🟢 (B) intensity of light
🟠 (C) work function
🔴 (D) stopping potential
Answer: (B) intensity of light
Year: 2024 | Apr Shift 2
Question 7: The velocity of electron of de Broglie wavelength λ is
🔵 (A) h/mλ
🟢 (B) λh/m
🟠 (C) mλ/h
🔴 (D) hλ/m
Answer: (A) h/mλ
Year: 2023 | Jan Shift 1
Question 8: The unit of Planck’s constant is
🔵 (A) J/s
🟢 (B) Js
🟠 (C) J/m
🔴 (D) J·m/s
Answer: (B) Js
Year: 2023 | Apr Shift 1
Question 9: When frequency of incident light is less than threshold frequency, photoelectric emission
🔵 (A) takes place at higher intensity
🟢 (B) does not take place
🟠 (C) depends on temperature
🔴 (D) depends on pressure
Answer: (B) does not take place
Year: 2023 | Apr Shift 2
Question 10: The wave associated with matter is
🔵 (A) electromagnetic wave
🟢 (B) de Broglie wave
🟠 (C) stationary wave
🔴 (D) none
Answer: (B) de Broglie wave
Year: 2022 | Jul Shift 1
Question 11: The work function of sodium is 2.3 eV. The threshold wavelength is
🔵 (A) 540 nm
🟢 (B) 620 nm
🟠 (C) 310 nm
🔴 (D) 1240 nm
Answer: (A) 540 nm
Year: 2022 | Jun Shift 2
Question 12: A photon of 10 eV strikes a metal surface with work function 4 eV. Maximum KE of emitted electron is
🔵 (A) 10 eV
🟢 (B) 6 eV
🟠 (C) 4 eV
🔴 (D) 14 eV
Answer: (B) 6 eV
Year: 2022 | Jun Shift 1
Question 13: The de Broglie wavelength of a particle varies inversely with
🔵 (A) energy
🟢 (B) velocity
🟠 (C) momentum
🔴 (D) all of these
Answer: (C) momentum
Year: 2021 | Feb Shift 1
Question 14: The frequency of light required to eject electron from metal surface depends on
🔵 (A) work function
🟢 (B) intensity
🟠 (C) current
🔴 (D) wavelength
Answer: (A) work function
Year: 2021 | Mar Shift 1
Question 15: The relation between wavelength λ, Planck’s constant h, and momentum p is
🔵 (A) λ = p/h
🟢 (B) λ = h/p
🟠 (C) λ = h×p
🔴 (D) λ = hp
Answer: (B) λ = h/p
Year: 2021 | Jul Shift 1
Question 16: The stopping potential in photoelectric effect is related to maximum KE by
🔵 (A) eVs = KEmax
🟢 (B) eVs = hf
🟠 (C) eVs = ϕ
🔴 (D) eVs = hf − ϕ/2
Answer: (A) eVs = KEmax
Year: 2021 | Mar Shift 2
Question 17: In de Broglie hypothesis, wavelength is inversely proportional to
🔵 (A) charge
🟢 (B) mass and velocity
🟠 (C) potential
🔴 (D) energy
Answer: (B) mass and velocity
Year: 2020 | Jan Shift 1
Question 18: Which experiment established wave nature of electrons?
🔵 (A) Davisson–Germer experiment
🟢 (B) Photoelectric effect
🟠 (C) Compton scattering
🔴 (D) Millikan’s oil drop
Answer: (A) Davisson–Germer experiment
Year: 2020 | Sept Shift 1
Question 19: In photoelectric effect, emission of electrons is instantaneous because
🔵 (A) energy of photon is quantized
🟢 (B) photon velocity is high
🟠 (C) electron is light
🔴 (D) threshold is zero
Answer: (A) energy of photon is quantized
Year: 2020 | Sept Shift 2
Question 20: The particle nature of light is supported by
🔵 (A) diffraction
🟢 (B) interference
🟠 (C) photoelectric effect
🔴 (D) polarization
Answer: (C) photoelectric effect
Year: 2019 | Apr Shift 1
Question 21: Which effect cannot be explained by wave theory of light?
🔵 (A) Interference
🟢 (B) Diffraction
🟠 (C) Photoelectric effect
🔴 (D) Polarization
Answer: (C) Photoelectric effect
Year: 2019 | Jan Shift 1
Question 22: The unit of work function is
🔵 (A) Joule
🟢 (B) eV
🟠 (C) both A and B
🔴 (D) erg
Answer: (C) both A and B
Year: 2019 | Apr Shift 2
Question 23: A proton and an electron have same momentum. Their de Broglie wavelengths are
🔵 (A) same
🟢 (B) different, electron larger
🟠 (C) different, proton larger
🔴 (D) cannot say
Answer: (A) same
Year: 2018
Question 24: Which of the following has maximum penetrating power?
🔵 (A) α-particles
🟢 (B) β-particles
🟠 (C) γ-rays
🔴 (D) electrons
Answer: (C) γ-rays
Year: 2018
Question 25: The energy of photon is related to its wavelength by
🔵 (A) E = hc/λ
🟢 (B) E = λ/hc
🟠 (C) E = hλc
🔴 (D) E = hcλ
Answer: (A) E = hc/λ
Year: 2018
Question 26: The momentum of a photon of wavelength λ is
🔵 (A) h/λ
🟢 (B) hc/λ
🟠 (C) λ/h
🔴 (D) λ/hc
Answer: (A) h/λ
Year: 2017
Question 27: The photoelectric effect supports which nature of light?
🔵 (A) Wave nature
🟢 (B) Particle nature
🟠 (C) Both A and B
🔴 (D) None
Answer: (B) Particle nature
Year: 2017
Question 28: A photon of 5 eV strikes a metal of work function 3 eV. The maximum kinetic energy of photoelectron is
🔵 (A) 5 eV
🟢 (B) 2 eV
🟠 (C) 3 eV
🔴 (D) 8 eV
Answer: (B) 2 eV
Year: 2017
Question 29: The concept of matter waves was given by
🔵 (A) Einstein
🟢 (B) Planck
🟠 (C) de Broglie
🔴 (D) Bohr
Answer: (C) de Broglie
Year: 2016
Question 30: The wave nature of electrons is confirmed by
🔵 (A) Compton effect
🟢 (B) Davisson–Germer experiment
🟠 (C) Zeeman effect
🔴 (D) Photoelectric effect
Answer: (B) Davisson–Germer experiment
Year: 2016
Question 31: In photoelectric effect, if frequency of incident light is increased, then stopping potential
🔵 (A) decreases
🟢 (B) increases
🟠 (C) remains constant
🔴 (D) none
Answer: (B) increases
Year: 2016
Question 32: For a particle of mass m moving with velocity v, de Broglie wavelength is
🔵 (A) h/mv
🟢 (B) hv/m
🟠 (C) mv/h
🔴 (D) hm/v
Answer: (A) h/mv
Year: 2015
Question 33: The quantity “work function” is
🔵 (A) energy
🟢 (B) power
🟠 (C) force
🔴 (D) intensity
Answer: (A) energy
Year: 2015
Question 34: The photocurrent in a photoelectric experiment increases with increase of
🔵 (A) frequency of light
🟢 (B) intensity of light
🟠 (C) work function
🔴 (D) stopping potential
Answer: (B) intensity of light
Year: 2015
Question 35: The photoelectric effect is instantaneous because
🔵 (A) photon delivers energy in lump
🟢 (B) electrons are light
🟠 (C) metal is conducting
🔴 (D) intensity is high
Answer: (A) photon delivers energy in lump
Year: 2014
Question 36: The maximum KE of photoelectrons depends on
🔵 (A) intensity
🟢 (B) frequency of incident light
🟠 (C) angle of incidence
🔴 (D) emission area
Answer: (B) frequency of incident light
Year: 2014
Question 37: Which of the following particles have both wave and particle nature?
🔵 (A) Photon only
🟢 (B) Electron only
🟠 (C) Proton only
🔴 (D) All matter particles
Answer: (D) All matter particles
Year: 2014
Question 38: The stopping potential in photoelectric effect depends on
🔵 (A) intensity of incident light
🟢 (B) frequency of incident light
🟠 (C) number of photoelectrons
🔴 (D) area of cathode
Answer: (B) frequency of incident light
Year: 2013
Question 39: In photoelectric effect, maximum KE of emitted electron is zero when
🔵 (A) hf < ϕ 🟢 (B) hf = ϕ 🟠 (C) hf > ϕ
🔴 (D) none
Answer: (B) hf = ϕ
Year: 2013
Question 40: Which one is not explained by particle nature of light?
🔵 (A) Photoelectric effect
🟢 (B) Compton effect
🟠 (C) Diffraction
🔴 (D) Pair production
Answer: (C) Diffraction
Year: 2013
Question 41: The relation between energy of photon and frequency is
🔵 (A) E = hf
🟢 (B) E = h/f
🟠 (C) E = hf²
🔴 (D) E = h/λ
Answer: (A) E = hf
Year: 2012 (AIEEE)
Question 42: The work function of a material is 4.1 eV. Threshold frequency is approximately
🔵 (A) 9.9 × 10¹⁴ Hz
🟢 (B) 10 × 10¹² Hz
🟠 (C) 3 × 10¹² Hz
🔴 (D) 5 × 10¹⁰ Hz
Answer: (A) 9.9 × 10¹⁴ Hz
Year: 2012 (AIEEE)
Question 43: Photoelectric effect was explained by
🔵 (A) Einstein
🟢 (B) Planck
🟠 (C) Millikan
🔴 (D) Hertz
Answer: (A) Einstein
Year: 2011 (AIEEE)
Question 44: The experiment which established quantum nature of light is
🔵 (A) Davisson–Germer
🟢 (B) Photoelectric effect
🟠 (C) Double slit
🔴 (D) Diffraction grating
Answer: (B) Photoelectric effect
Year: 2011 (AIEEE)
Question 45: The de Broglie wavelength of a 1 keV electron is about
🔵 (A) 0.04 nm
🟢 (B) 1.2 nm
🟠 (C) 0.12 nm
🔴 (D) 12.2 nm
Answer: (A) 0.04 nm
Year: 2010 (AIEEE)
Question 46: The photocurrent depends upon
🔵 (A) frequency only
🟢 (B) intensity only
🟠 (C) stopping potential only
🔴 (D) both frequency & intensity
Answer: (B) intensity only
Year: 2010 (AIEEE)
Question 47: The wave nature of matter is significant for
🔵 (A) large bodies
🟢 (B) electrons
🟠 (C) planets
🔴 (D) stars
Answer: (B) electrons
Year: 2009 (AIEEE)
Question 48: The maximum kinetic energy of photoelectron is given by
🔵 (A) KEmax = hf
🟢 (B) KEmax = hf − ϕ
🟠 (C) KEmax = ϕ − hf
🔴 (D) KEmax = h/λ
Answer: (B) KEmax = hf − ϕ
Year: 2009 (AIEEE)
Question 49: In photoelectric effect, emission takes place only if
🔵 (A) frequency > threshold
🟢 (B) frequency < threshold 🟠 (C) intensity > threshold
🔴 (D) wavelength > threshold
Answer: (A) frequency > threshold
Year: 2008 (AIEEE)
Question 50: The scientist who first observed photoelectric effect was
🔵 (A) Hertz
🟢 (B) Einstein
🟠 (C) Planck
🔴 (D) Bohr
Answer: (A) Hertz
Year: 2007 (AIEEE)
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
Question 1: The stopping potential in a photoelectric experiment depends on
🔵 (A) intensity of light
🟢 (B) frequency of light
🟠 (C) work function
🔴 (D) both frequency and work function
Answer: (D) both frequency and work function
Year: 2023 | Paper 1
Question 2: According to Einstein’s photoelectric equation, the slope of the graph of stopping potential vs frequency is
🔵 (A) h
🟢 (B) h/e
🟠 (C) e/h
🔴 (D) 1/h
Answer: (B) h/e
Year: 2023 | Paper 1
Question 3: The photoelectric effect supports
🔵 (A) wave nature of light
🟢 (B) particle nature of light
🟠 (C) both
🔴 (D) neither
Answer: (B) particle nature of light
Year: 2022 | Paper 1
Question 4: In photoelectric emission, the kinetic energy of emitted electrons depends upon
🔵 (A) intensity of light
🟢 (B) frequency of incident radiation
🟠 (C) angle of incidence
🔴 (D) duration of exposure
Answer: (B) frequency of incident radiation
Year: 2022 | Paper 1
Question 5: Work function of a metal is 2 eV. Threshold wavelength is
🔵 (A) 310 nm
🟢 (B) 620 nm
🟠 (C) 1240 nm
🔴 (D) 2480 nm
Answer: (B) 620 nm
Year: 2021 | Paper 1
Question 6: If intensity of incident light is doubled in a photoelectric experiment, then
🔵 (A) KEmax doubles
🟢 (B) number of photoelectrons doubles
🟠 (C) stopping potential doubles
🔴 (D) work function doubles
Answer: (B) number of photoelectrons doubles
Year: 2021 | Paper 1
Question 7: de Broglie wavelength of an electron of momentum p is
🔵 (A) h/p
🟢 (B) p/h
🟠 (C) hcp
🔴 (D) e/p
Answer: (A) h/p
Year: 2020 | Paper 1
Question 8: The momentum of a photon of wavelength λ is
🔵 (A) h/λ
🟢 (B) λ/h
🟠 (C) hc/λ
🔴 (D) 1/hλ
Answer: (A) h/λ
Year: 2020 | Paper 1
Question 9: If frequency of incident light is below threshold frequency, photoelectric emission
🔵 (A) does not occur
🟢 (B) occurs slowly
🟠 (C) occurs with delay
🔴 (D) occurs with reduced intensity
Answer: (A) does not occur
Year: 2019 | Paper 1
Question 10: The maximum kinetic energy of photoelectrons is given by
🔵 (A) KEmax = hf
🟢 (B) KEmax = hf − φ
🟠 (C) KEmax = φ − hf
🔴 (D) KEmax = φ
Answer: (B) KEmax = hf − φ
Year: 2019 | Paper 1
Question 11: The graph of KEmax vs frequency is
🔵 (A) a parabola
🟢 (B) a straight line
🟠 (C) hyperbola
🔴 (D) circle
Answer: (B) a straight line
Year: 2018 | Paper 1
Question 12: A photocell is illuminated by monochromatic light of frequency greater than threshold. If intensity is doubled, then stopping potential
🔵 (A) doubles
🟢 (B) remains same
🟠 (C) halves
🔴 (D) becomes zero
Answer: (B) remains same
Year: 2018 | Paper 1
Question 13: The wavelength associated with an electron accelerated by potential V is proportional to
🔵 (A) 1/√V
🟢 (B) √V
🟠 (C) V
🔴 (D) 1/V
Answer: (A) 1/√V
Year: 2017 | Paper 1
Question 14: The photon momentum is related to energy E as
🔵 (A) p = E/c
🟢 (B) p = Ec
🟠 (C) p = c/E
🔴 (D) p = hE
Answer: (A) p = E/c
Year: 2017 | Paper 1
Question 15: Which radiation has maximum penetrating power?
🔵 (A) α-particles
🟢 (B) β-particles
🟠 (C) γ-rays
🔴 (D) protons
Answer: (C) γ-rays
Year: 2016 | Paper 1
Question 16: The photoelectric effect cannot be explained by
🔵 (A) quantum theory
🟢 (B) wave theory of light
🟠 (C) photon concept
🔴 (D) Planck’s quantum hypothesis
Answer: (B) wave theory of light
Year: 2016 | Paper 1
Question 17: The dual nature of matter was experimentally confirmed by
🔵 (A) Hertz
🟢 (B) Davisson and Germer
🟠 (C) Planck
🔴 (D) Einstein
Answer: (B) Davisson and Germer
Year: 2015 | Paper 1
Question 18: The energy of a photon of wavelength λ is
🔵 (A) hc/λ
🟢 (B) h/λ
🟠 (C) λ/h
🔴 (D) λc/h
Answer: (A) hc/λ
Year: 2023 | Paper 2
Question 19: In photoelectric emission, stopping potential is independent of
🔵 (A) frequency of radiation
🟢 (B) intensity of radiation
🟠 (C) work function of material
🔴 (D) Planck’s constant
Answer: (B) intensity of radiation
Year: 2023 | Paper 2
Question 20: The maximum wavelength of light that can cause photoelectric emission is called
🔵 (A) de Broglie wavelength
🟢 (B) threshold wavelength
🟠 (C) Compton wavelength
🔴 (D) none
Answer: (B) threshold wavelength
Year: 2022 | Paper 2
Question 21: The slope of KEmax vs frequency graph gives
🔵 (A) work function
🟢 (B) Planck’s constant
🟠 (C) h/e
🔴 (D) e/h
Answer: (B) Planck’s constant
Year: 2022 | Paper 2
Question 22: A photocathode has work function 2.5 eV. Threshold frequency is
🔵 (A) 1.2×10^14 Hz
🟢 (B) 6.0×10^14 Hz
🟠 (C) 12×10^14 Hz
🔴 (D) 24×10^14 Hz
Answer: (B) 6.0×10^14 Hz
Year: 2021 | Paper 2
Question 23: If intensity of incident light is halved, then photoelectric current becomes
🔵 (A) doubled
🟢 (B) halved
🟠 (C) zero
🔴 (D) unchanged
Answer: (B) halved
Year: 2021 | Paper 2
Question 24: de Broglie wavelength of an electron accelerated by potential V is
🔵 (A) h/√(2meV)
🟢 (B) h/√(meV)
🟠 (C) h/√(2V)
🔴 (D) √(2hV/me)
Answer: (A) h/√(2meV)
Year: 2020 | Paper 2
Question 25: Momentum of photon of frequency f is
🔵 (A) hf/c
🟢 (B) hc/f
🟠 (C) f/hc
🔴 (D) fλ
Answer: (A) hf/c
Year: 2020 | Paper 2
Question 26: The minimum energy required to emit an electron from metal surface is called
🔵 (A) kinetic energy
🟢 (B) work function
🟠 (C) threshold energy
🔴 (D) potential energy
Answer: (B) work function
Year: 2019 | Paper 2
Question 27: If frequency of incident light equals threshold frequency, then KEmax of photoelectron is
🔵 (A) zero
🟢 (B) hf0
🟠 (C) eV0
🔴 (D) infinite
Answer: (A) zero
Year: 2019 | Paper 2
Question 28: Wavelength of electron moving with velocity v is
🔵 (A) h/p
🟢 (B) h/mv
🟠 (C) h/v
🔴 (D) hv/m
Answer: (B) h/mv
Year: 2018 | Paper 2
Question 29: The concept of matter waves was proposed by
🔵 (A) Einstein
🟢 (B) Planck
🟠 (C) de Broglie
🔴 (D) Davisson
Answer: (C) de Broglie
Year: 2018 | Paper 2
Question 30: Compton effect demonstrates
🔵 (A) particle nature of light
🟢 (B) wave nature of light
🟠 (C) both
🔴 (D) none
Answer: (A) particle nature of light
Year: 2017 | Paper 2
Question 31: If incident radiation has frequency less than threshold frequency, then
🔵 (A) no emission occurs
🟢 (B) emission occurs with low KE
🟠 (C) emission occurs with delay
🔴 (D) emission intensity reduces
Answer: (A) no emission occurs
Year: 2017 | Paper 2
Question 32: Photoelectric effect was explained by
🔵 (A) Einstein
🟢 (B) Planck
🟠 (C) Millikan
🔴 (D) Hertz
Answer: (A) Einstein
Year: 2016 | Paper 2
Question 33: Which experiment confirmed the wave nature of electrons?
🔵 (A) Davisson–Germer experiment
🟢 (B) Millikan oil drop
🟠 (C) Rutherford scattering
🔴 (D) Michelson–Morley
Answer: (A) Davisson–Germer experiment
Year: 2016 | Paper 2
Question 34: The de Broglie wavelength associated with a proton of kinetic energy K is
🔵 (A) h/√(2mK)
🟢 (B) h/√(mK)
🟠 (C) hK/m
🔴 (D) K/hm
Answer: (A) h/√(2mK)
Year: 2015 | Paper 2
————————————————————————————————————————————————————————————————————————————
PRACTICE SETS FROM THIS LESSON
🔹 Part A — NEET Level (Q1–Q20)
✏️ Q1. Work function of a metal is 2 eV. Threshold wavelength is:
🔵 A) 620 nm
🟢 B) 540 nm
🔴 C) 310 nm
🟡 D) 1240 nm
✔️ Answer: A) 620 nm
✏️ Q2. If the intensity of incident light doubles, the photoelectric current:
🔵 A) Becomes half
🟢 B) Doubles
🔴 C) Becomes four times
🟡 D) Remains same
✔️ Answer: B) Doubles
✏️ Q3. Which graph represents KE_max vs frequency correctly?
🔵 A) Straight line parallel to frequency axis
🟢 B) Parabola
🔴 C) Straight line with positive slope
🟡 D) Hyperbola
✔️ Answer: C) Straight line with positive slope
✏️ Q4. Stopping potential depends upon:
🔵 A) Intensity only
🟢 B) Frequency only
🔴 C) Both intensity and frequency
🟡 D) Neither
✔️ Answer: B) Frequency only
✏️ Q5. For photoelectric emission, if frequency < threshold, then:
🔵 A) Electrons are emitted with less energy
🟢 B) Electrons are emitted with delay
🔴 C) No electrons emitted
🟡 D) Current decreases slowly
✔️ Answer: C) No electrons emitted
✏️ Q6. Energy of a photon of λ = 400 nm:
🔵 A) 3.1 eV
🟢 B) 1.55 eV
🔴 C) 4.2 eV
🟡 D) 6.2 eV
✔️ Answer: A) 3.1 eV
✏️ Q7. Which phenomenon proves particle nature of light?
🔵 A) Diffraction
🟢 B) Interference
🔴 C) Photoelectric effect
🟡 D) Polarisation
✔️ Answer: C) Photoelectric effect
✏️ Q8. The SI unit of work function is:
🔵 A) J
🟢 B) eV
🔴 C) J·s
🟡 D) V
✔️ Answer: A) J
✏️ Q9. Momentum of a photon of wavelength λ is:
🔵 A) hc/λ
🟢 B) λ/h
🔴 C) h/λ
🟡 D) E·c
✔️ Answer: C) h/λ
✏️ Q10. Which metal has lowest work function among the following?
🔵 A) Cesium
🟢 B) Sodium
🔴 C) Aluminium
🟡 D) Copper
✔️ Answer: A) Cesium
✏️ Q11. Photoelectric effect is instantaneous because:
🔵 A) Photons are heavy
🟢 B) Electrons store energy
🔴 C) Energy transfer is one-to-one
🟡 D) Wavefronts accelerate emission
✔️ Answer: C) Energy transfer is one-to-one
✏️ Q12. Stopping potential for electrons emitted by 2 eV photons from a metal of 1.2 eV work function is:
🔵 A) 0.8 V
🟢 B) 1.2 V
🔴 C) 2 V
🟡 D) 0.6 V
✔️ Answer: A) 0.8 V
✏️ Q13. Which constant is determined by slope of V–f graph?
🔵 A) Planck’s constant
🟢 B) Charge of electron
🔴 C) Work function
🟡 D) Speed of light
✔️ Answer: A) Planck’s constant
✏️ Q14. Wavelength of an electron accelerated by 100 eV is approximately:
🔵 A) 0.12 nm
🟢 B) 0.38 nm
🔴 C) 1.2 nm
🟡 D) 3.1 nm
✔️ Answer: A) 0.12 nm
✏️ Q15. A photon has momentum 1×10^-27 kg·m/s. Its wavelength is:
🔵 A) 0.66 nm
🟢 B) 6.6×10^-7 m
🔴 C) 1×10^-9 m
🟡 D) 3×10^8 m
✔️ Answer: B) 6.6×10^-7 m
✏️ Q16. Davisson–Germer experiment demonstrated:
🔵 A) Photon momentum
🟢 B) Electron diffraction
🔴 C) Pair production
🟡 D) Compton scattering
✔️ Answer: B) Electron diffraction
✏️ Q17. If work function is 2.5 eV, threshold frequency is:
🔵 A) 4×10^14 Hz
🟢 B) 6×10^14 Hz
🔴 C) 8×10^14 Hz
🟡 D) 1.2×10^15 Hz
✔️ Answer: B) 6×10^14 Hz
✏️ Q18. When frequency increases, slope of KE vs f graph:
🔵 A) Increases
🟢 B) Decreases
🔴 C) Remains constant
🟡 D) Zero
✔️ Answer: C) Remains constant
✏️ Q19. If intensity of light increases, KE_max of photoelectrons:
🔵 A) Increases
🟢 B) Decreases
🔴 C) Remains constant
🟡 D) Becomes zero
✔️ Answer: C) Remains constant
✏️ Q20. A photon of 5 eV falls on a surface of 3 eV work function. KE_max = ?
🔵 A) 2 eV
🟢 B) 5 eV
🔴 C) 8 eV
🟡 D) 3 eV
✔️ Answer: A) 2 eV
🔹 Part B — JEE Main Level (Q21–Q40)
✏️ Q21. The kinetic energy of an electron is 150 eV. Its de Broglie wavelength is:
🔵 A) 0.1 nm
🟢 B) 0.05 nm
🔴 C) 0.2 nm
🟡 D) 1.2 nm
✔️ Answer: A) 0.1 nm
✏️ Q22. Photoelectric current vs intensity graph is:
🔵 A) Linear
🟢 B) Parabola
🔴 C) Constant
🟡 D) Logarithmic
✔️ Answer: A) Linear
✏️ Q23. For a photon, relation between energy and momentum is:
🔵 A) E = pc
🟢 B) E = p^2c
🔴 C) E = p/c
🟡 D) E = p^2/2m
✔️ Answer: A) E = pc
✏️ Q24. Which property is common to both photons and electrons?
🔵 A) Rest mass
🟢 B) Charge
🔴 C) Momentum
🟡 D) Spin
✔️ Answer: C) Momentum
✏️ Q25. The dimensional formula of Planck’s constant is:
🔵 A) ML^2T^-1
🟢 B) ML^2T^-2
🔴 C) ML^3T^-2
🟡 D) MLT^-2
✔️ Answer: A) ML^2T^-1
✏️ Q26. In photoelectric effect, number of emitted electrons depends on:
🔵 A) Frequency
🟢 B) Intensity
🔴 C) Work function
🟡 D) Stopping potential
✔️ Answer: B) Intensity
✏️ Q27. The electron of KE = 1 eV has velocity:
🔵 A) 5.9×10^5 m/s
🟢 B) 1.2×10^6 m/s
🔴 C) 3×10^8 m/s
🟡 D) 2×10^4 m/s
✔️ Answer: A) 5.9×10^5 m/s
✏️ Q28. The maximum velocity of emitted photoelectron when frequency is 2× threshold frequency is:
🔵 A) √(hν/m)
🟢 B) √(hν − ϕ)
🔴 C) √(hν/2m)
🟡 D) √(hν/m − 2ϕ)
✔️ Answer: B) √(hν − ϕ)
✏️ Q29. Work function of a metal is 4 eV. Threshold wavelength = ?
🔵 A) 310 nm
🟢 B) 540 nm
🔴 C) 1240 nm
🟡 D) 600 nm
✔️ Answer: A) 310 nm
✏️ Q30. A photoelectron of KE = 4 eV has de Broglie wavelength:
🔵 A) 0.62 nm
🟢 B) 0.2 nm
🔴 C) 1.2 nm
🟡 D) 3.0 nm
✔️ Answer: B) 0.2 nm
✏️ Q31. de Broglie hypothesis associates:
🔵 A) Wave with radiation only
🟢 B) Wave with matter only
🔴 C) Wave with both matter and radiation
🟡 D) None
✔️ Answer: C) Wave with both matter and radiation
✏️ Q32. Which of the following proves dual nature of light?
🔵 A) Interference
🟢 B) Diffraction
🔴 C) Photoelectric effect
🟡 D) Both A and C
✔️ Answer: D) Both A and C
✏️ Q33. Cut-off wavelength for a surface is 600 nm. Work function = ?
🔵 A) 2.06 eV
🟢 B) 1.8 eV
🔴 C) 3.1 eV
🟡 D) 2.5 eV
✔️ Answer: A) 2.06 eV
✏️ Q34. Which experiment first confirmed de Broglie hypothesis?
🔵 A) Compton
🟢 B) Davisson–Germer
🔴 C) Rutherford scattering
🟡 D) Young’s double slit
✔️ Answer: B) Davisson–Germer
✏️ Q35. For λ = 500 nm, photon momentum = ?
🔵 A) 1.3×10^-27 kg·m/s
🟢 B) 2.0×10^-27 kg·m/s
🔴 C) 6.6×10^-34 kg·m/s
🟡 D) 3.0×10^8 kg·m/s
✔️ Answer: A) 1.3×10^-27 kg·m/s
✏️ Q36. If V–f graph intercepts frequency axis at 6×10^14 Hz, work function is:
🔵 A) 2.48 eV
🟢 B) 3.98 eV
🔴 C) 1.6 eV
🟡 D) 4.2 eV
✔️ Answer: B) 3.98 eV
✏️ Q37. Electron accelerated through 25 V has λ = ?
🔵 A) 0.25 nm
🟢 B) 0.78 nm
🔴 C) 1.2 nm
🟡 D) 2.0 nm
✔️ Answer: B) 0.78 nm
✏️ Q38. Stopping potential becomes zero if:
🔵 A) Frequency < threshold
🟢 B) Intensity increases
🔴 C) Work function decreases
🟡 D) KE increases
✔️ Answer: A) Frequency < threshold
✏️ Q39. Which of these supports corpuscular nature of light?
🔵 A) Interference
🟢 B) Diffraction
🔴 C) Photoelectric effect
🟡 D) Polarisation
✔️ Answer: C) Photoelectric effect
✏️ Q40. Energy of electron accelerated by 1 kV:
🔵 A) 100 eV
🟢 B) 1000 eV
🔴 C) 2000 eV
🟡 D) 500 eV
✔️ Answer: B) 1000 eV
🔹 Part C — JEE Advanced Level (Q41–Q50)
✏️ Q41. A photon of λ = 300 nm strikes a metal with ϕ = 2 eV. Find KE_max.
🔵 A) 2.1 eV
🟢 B) 4.13 eV
🔴 C) 6.2 eV
🟡 D) 1.2 eV
✔️ Answer: A) 2.1 eV
✏️ Q42. In Davisson–Germer experiment, maximum diffraction occurs at 54 V. λ of electron is close to:
🔵 A) 0.165 nm
🟢 B) 0.55 nm
🔴 C) 0.85 nm
🟡 D) 1.2 nm
✔️ Answer: A) 0.165 nm
✏️ Q43. If work function is 5 eV, threshold wavelength is:
🔵 A) 250 nm
🟢 B) 310 nm
🔴 C) 620 nm
🟡 D) 1240 nm
✔️ Answer: A) 250 nm
✏️ Q44. A beam of photons each 4 eV falls on a surface of 2.5 eV work function. If 10^15 photons fall, total emitted KE is:
🔵 A) 2.5 J
🟢 B) 1.5 J
🔴 C) 0.6 J
🟡 D) 0.24 J
✔️ Answer: B) 1.5 J
✏️ Q45. The momentum of an electron with KE = 2 eV is:
🔵 A) 7.6×10^-25 kg·m/s
🟢 B) 2.4×10^-24 kg·m/s
🔴 C) 3.0×10^-26 kg·m/s
🟡 D) 1.2×10^-25 kg·m/s
✔️ Answer: A) 7.6×10^-25 kg·m/s
✏️ Q46. A light source emits photons at rate of 10^20 photons/s of λ = 500 nm. Power output is:
🔵 A) 40 W
🟢 B) 30 W
🔴 C) 20 W
🟡 D) 10 W
✔️ Answer: A) 40 W
✏️ Q47. Electron accelerated through 100 V has de Broglie λ = ?
🔵 A) 0.123 nm
🟢 B) 0.25 nm
🔴 C) 0.62 nm
🟡 D) 0.012 nm
✔️ Answer: A) 0.123 nm
✏️ Q48. Work function of sodium = 2.3 eV. Threshold frequency = ?
🔵 A) 5.56×10^14 Hz
🟢 B) 4.2×10^14 Hz
🔴 C) 6.2×10^14 Hz
🟡 D) 3.0×10^15 Hz
✔️ Answer: A) 5.56×10^14 Hz
✏️ Q49. If λ = 350 nm radiation falls on a metal of ϕ = 2 eV, KE_max = ?
🔵 A) 1.54 eV
🟢 B) 2.2 eV
🔴 C) 3.1 eV
🟡 D) 0.6 eV
✔️ Answer: A) 1.54 eV
✏️ Q50. In a photoelectric experiment, cut-off potential is 3 V. Maximum KE of electrons is:
🔵 A) 3 eV
🟢 B) 6 eV
🔴 C) 1.5 eV
🟡 D) 0.5 eV
✔️ Answer: A) 3 eV
————————————————————————————————————————————————————————————————————————————
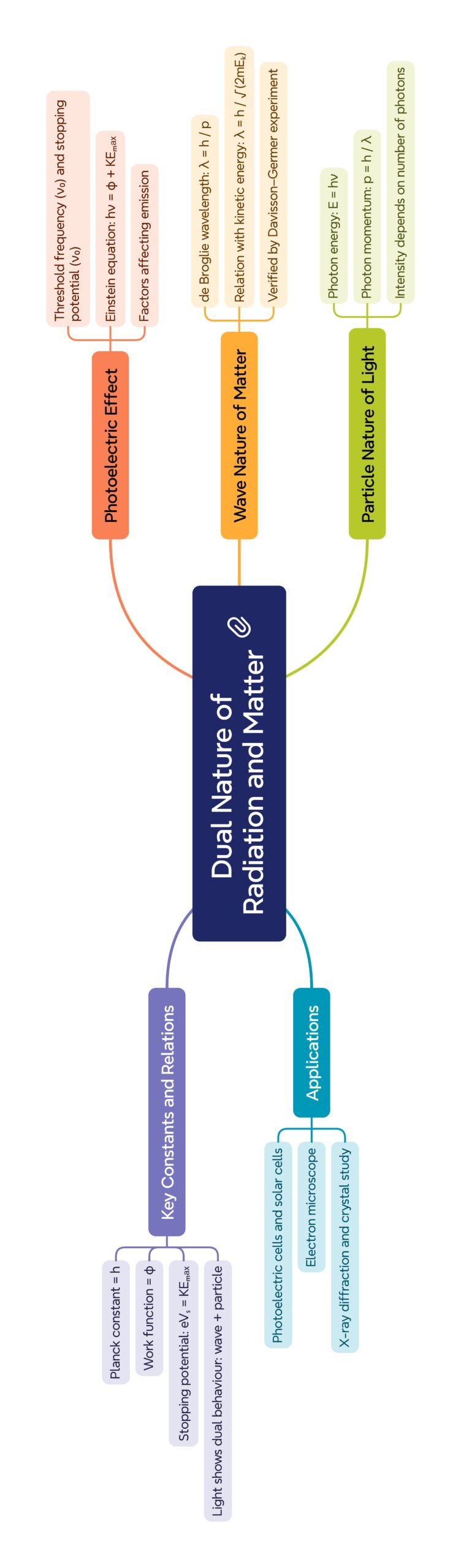
MIND MAP
————————————————————————————————————————————————————————————————————————————
