Class 12 : Chemistry (English) – Chapter 7: Alcohols, Phenols and Ethers
EXPLANATION & SUMMARY
Introduction
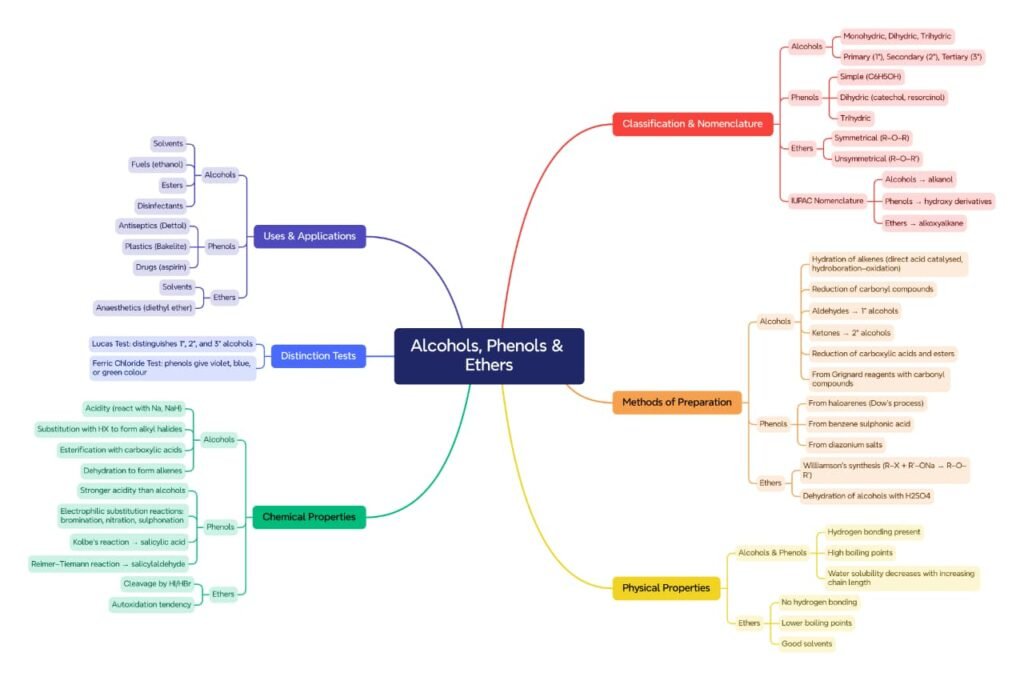
This chapter deals with three important classes of organic compounds: alcohols, phenols, and ethers. They are distinguished by the presence of the hydroxyl group (−OH) in alcohols and phenols, and the alkoxy group (−OR) in ethers. These compounds are significant in both biological and industrial contexts.
Classification and Nomenclature
Alcohols are compounds where one or more hydroxyl (−OH) groups are attached to aliphatic carbon chains. Based on the number of hydroxyl groups, alcohols are classified as:
Monohydric (one −OH group)
Dihydric (two −OH groups)
Trihydric (three −OH groups)
Depending on the carbon to which −OH is attached:
Primary alcohol (1°): −OH on a carbon bonded to one alkyl group
Secondary alcohol (2°): −OH on a carbon bonded to two alkyl groups
Tertiary alcohol (3°): −OH on a carbon bonded to three alkyl groups
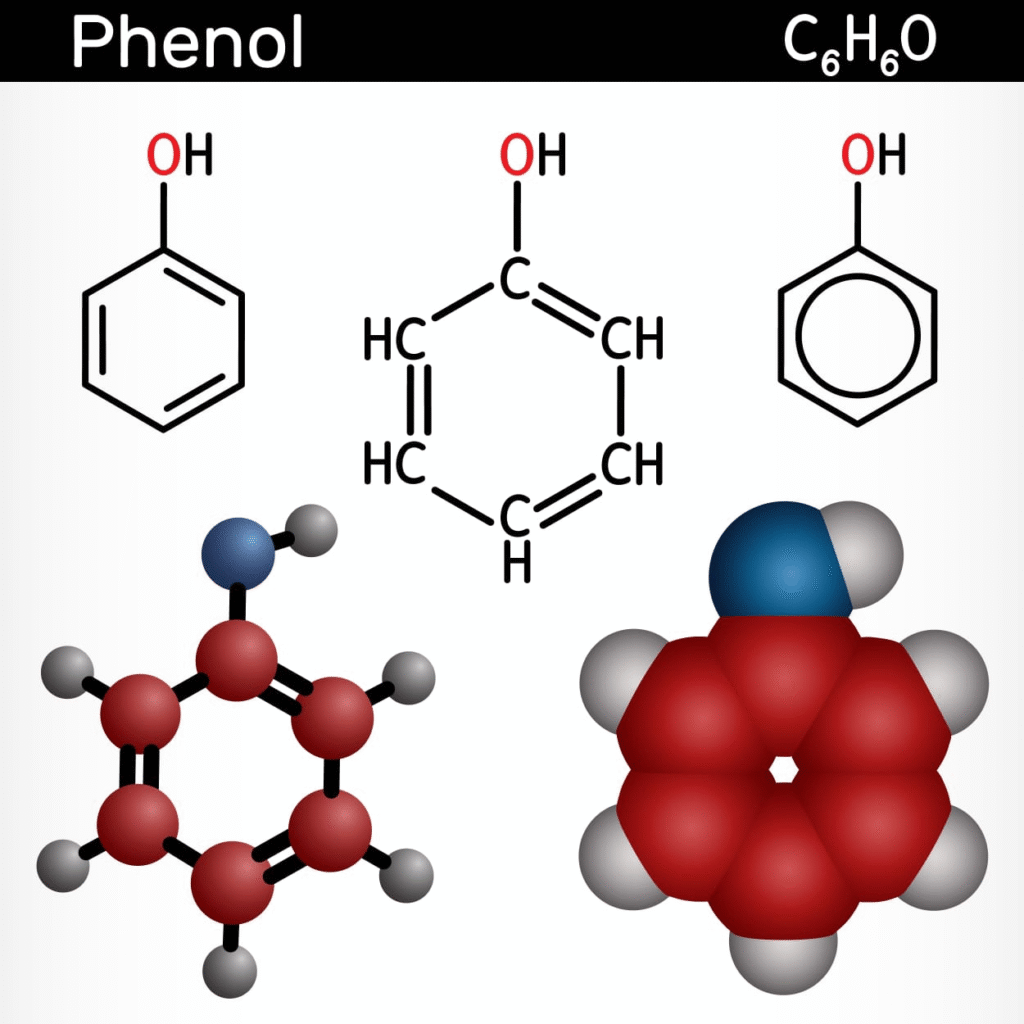
Phenols are compounds where the −OH group is directly attached to an aromatic ring.

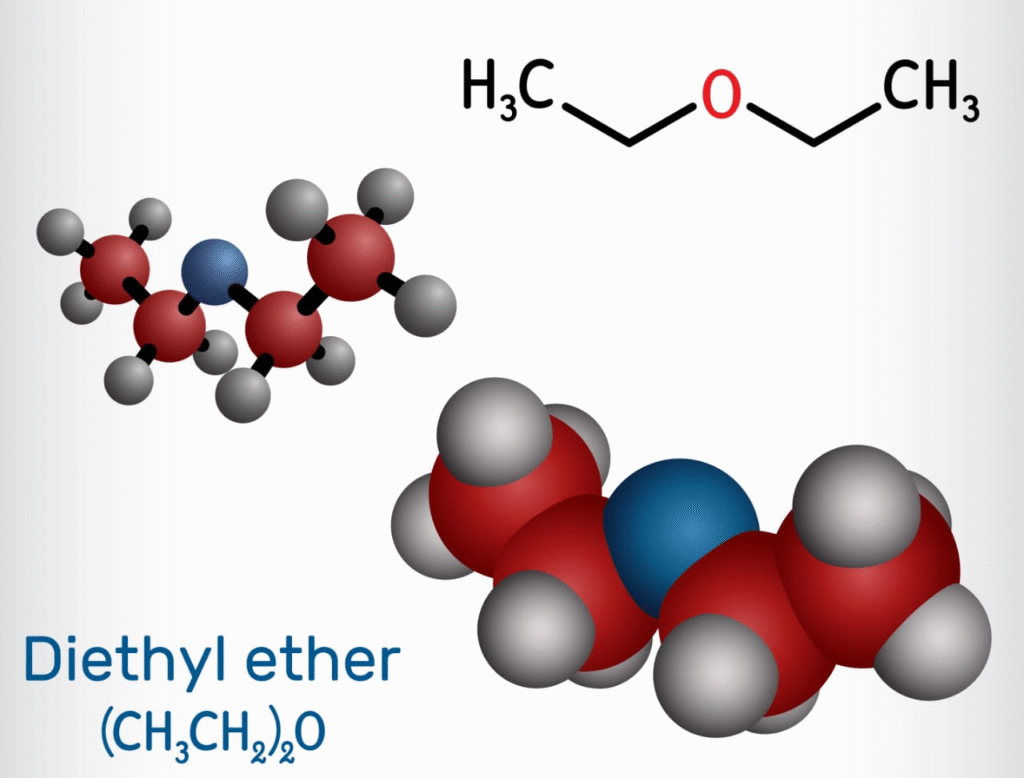
Ethers have the structure R−O−R′, where R and R′ can be alkyl or aryl groups.
IUPAC Nomenclature:
Alcohols: Replace ‘-e’ of alkane with ‘-ol’ (e.g., Propanol)
Phenols: Named as hydroxy derivatives of benzene (e.g., 2-Methylphenol)
Ethers: The simpler alkyl group is treated as a substituent using the term ‘alkoxy’ (e.g., Methoxyethane)
Structure of Functional Groups
In alcohols and phenols, the oxygen atom is sp³ hybridised and forms two sigma bonds — one with hydrogen and one with carbon — with two lone pairs. This bent structure makes alcohols and phenols polar.
Ethers also have an sp³ hybridised oxygen atom. The C−O−C bond angle is slightly greater than the tetrahedral angle due to repulsion between the lone pairs.
Methods of Preparation
Alcohols:
From alkenes (hydration):
Acid-catalysed hydration of alkenes produces alcohols via electrophilic addition.
From carbonyl compounds:
Reduction of aldehydes → primary alcohols
Reduction of ketones → secondary alcohols
By hydrolysis of alkyl halides:
Alkyl halide + aqueous KOH → Alcohol + KX
From Grignard Reagents:
Reaction with aldehydes/ketones followed by hydrolysis
Phenols:

From benzene sulphonic acid:
Fused with NaOH → phenol
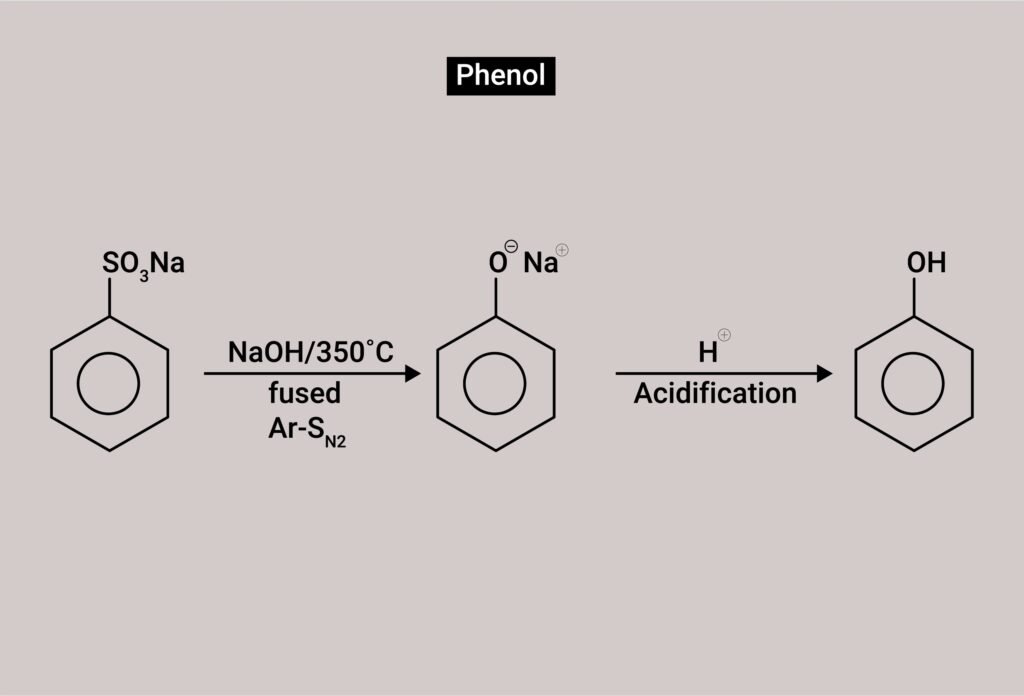
From diazonium salts:
Hydrolysis of diazonium salts → phenol
From Cumene:
Air oxidation of cumene followed by acid hydrolysis
Ethers:
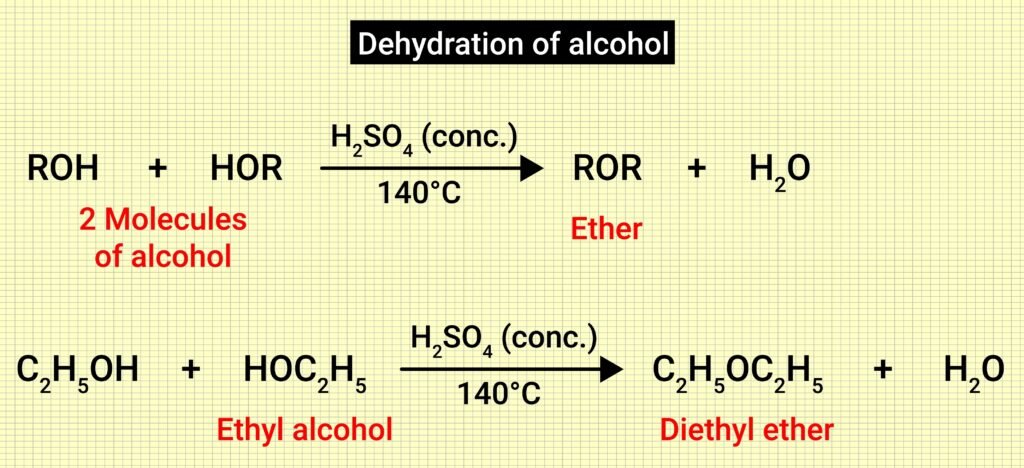
Dehydration of alcohols:
Symmetrical ethers formed using concentrated H₂SO₄ at 413 K
Williamson Ether Synthesis:
R−O⁻ + R′−X → R−O−R′ + X⁻
Suitable for synthesising unsymmetrical ethers
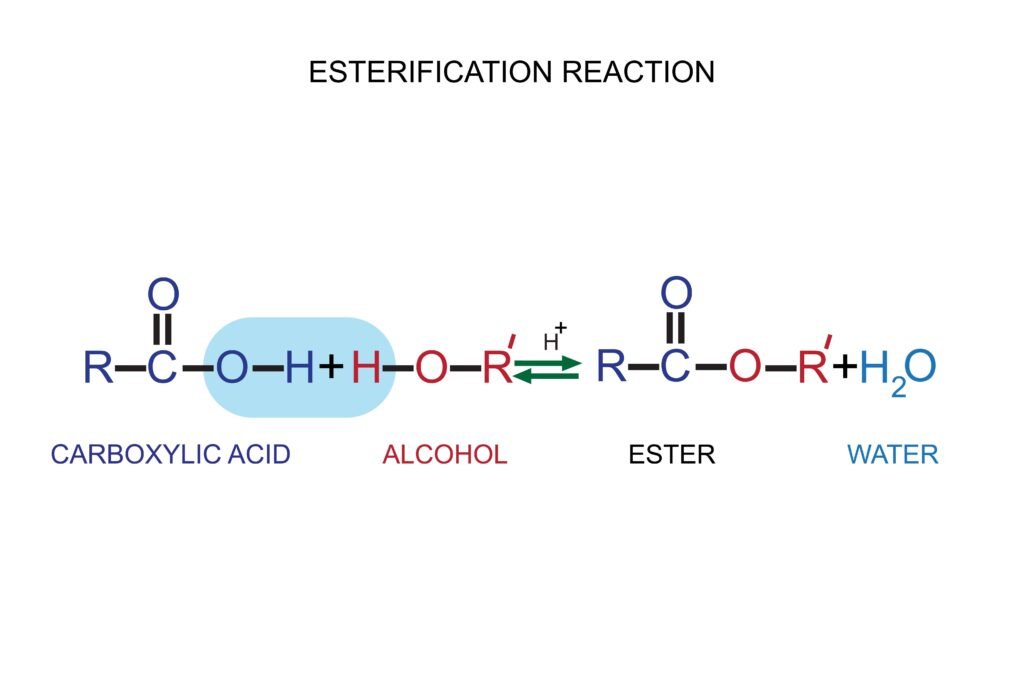
Physical Properties
Alcohols and phenols have higher boiling points than alkanes and ethers due to hydrogen bonding.
Solubility decreases with increase in alkyl group size.
Ethers have lower boiling points and are less soluble due to the absence of hydrogen bonding.
Chemical Reactions
Alcohols:
Reactions Involving −OH Group:
Acidic nature: Alcohols are weak acids. React with metals to form alkoxides.
Reaction with HX (Halogen acids):
ROH + HX → RX + H₂O
Reactions of Alcohol Molecule:
Dehydration:
ROH → Alkene + H₂O (in presence of acid and heat)
Oxidation:
1° alcohol → Aldehyde → Carboxylic acid
2° alcohol → Ketone
3° alcohol → Resistant to oxidation
Phenols:
Acidic Nature:
Phenols are more acidic than alcohols due to resonance stabilisation of phenoxide ion.
Electrophilic Substitution Reactions:
Halogenation: Phenol + Br₂ → 2,4,6-Tribromophenol
Nitration: Phenol + HNO₃ → o- and p-nitrophenol
Sulphonation: Phenol + H₂SO₄ → o- and p-sulphonic acid
Reimer-Tiemann Reaction:
Phenol + CHCl₃ + NaOH → Salicylaldehyde
Kolbe’s Reaction:
Phenol + CO₂ + NaOH → Salicylic acid
Ethers:
Cleavage by HX:
R−O−R′ + HX → RX + R′OH (mechanism varies for 1°/2°/3° alkyl groups)
Electrophilic Aromatic Substitution (for aryl ethers):
Similar to phenols but less reactive
Distinction between Alcohols, Phenols and Ethers
Property Alcohol Phenol Ether
−OH attached to sp³ carbon aromatic ring None (only −O− linkage)
Acidity Weak acid Stronger acid Neutral
H-bonding Yes Yes No
Reactivity with Na Yes Yes No
Oxidation Easy (except 3°) Difficult Not oxidised
Tests for Alcohols and Phenols
Lucas Test: Distinguishes between 1°, 2°, and 3° alcohols based on turbidity
Ferric Chloride Test: Phenol gives violet color
Victor Meyer Test: Differentiates primary, secondary, and tertiary alcohols
Uses and Applications
Alcohols: Solvents, fuels (e.g., ethanol), antiseptics

Phenols: Disinfectants, manufacture of resins and plastics
Ethers: Solvents (e.g., diethyl ether), anaesthetics
✍ SUMMARY (~300 words)
Alcohols, phenols, and ethers are classes of organic compounds containing oxygen.
Alcohols have the −OH group attached to aliphatic carbon; phenols to aromatic rings; ethers have an R−O−R′ structure.
Alcohols are classified into primary, secondary, and tertiary types. Phenols are further classified based on the number of −OH groups, and ethers as symmetrical or unsymmetrical.
Nomenclature is based on the IUPAC system, such as propan-2-ol for alcohols or methoxyethane for ethers.
Alcohols are prepared by hydration of alkenes, reduction of carbonyl compounds, and Grignard reaction. Phenols can be prepared from diazonium salts or cumene, and ethers via Williamson synthesis or dehydration of alcohols.
Alcohols and phenols are polar due to the −OH group and show hydrogen bonding, resulting in higher boiling points. Ethers do not hydrogen bond and are less soluble in water.
Alcohols react with metals, acids, and dehydrating agents. They can be oxidised to aldehydes, ketones, or acids. Phenols undergo electrophilic substitution easily and show characteristic reactions like Reimer-Tiemann and Kolbe’s reaction.
Ethers are relatively inert but cleave with strong acids like HI or HBr.
Lucas test, Ferric chloride test, and Victor Meyer test help distinguish alcohols and phenols.
These compounds are widely used in industry and daily life — alcohols as fuels and solvents, phenols in antiseptics and plastics, and ethers in pharmaceuticals and as solvents.
This chapter builds a strong foundation in understanding the behavior of oxygen-containing functional groups and is vital for both theoretical and practical applications in organic chemistry.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
7.1 Write IUPAC names of the following compounds:
(i) 2,2,4-Trimethylpentan-3-ol
Answer: Already the IUPAC name is given.
Structure:
CH₃
|
CH₃–C–CH₂–C(CH₃)₂–CH₃
|
OH
(ii) 5-Ethylheptane-2,4-diol
Answer: Already in IUPAC form.
Structure:
CH₃–CH₂–CH(OH)–CH(OH)–CH(CH₂CH₃)–CH₂–CH₃
(iii) Butane-2,3-diol
Structure: CH₃–CH(OH)–CH(OH)–CH₃
(iv) Propane-1,2,3-triol
Common name: Glycerol
Structure: CH₂OH–CHOH–CH₂OH
(v) 2-Methylphenol
Common name: o-Cresol
Structure: Benzene ring with OH at position 1, CH₃ at 2
(vi) 4-Methylphenol
Common name: p-Cresol
Structure: Benzene ring with OH at 1, CH₃ at 4
(vii) 2,5-Dimethylphenol
Structure: Benzene ring with OH at 1, CH₃ at 2 and 5
(viii) 2,6-Dimethylphenol
Structure: Benzene ring with OH at 1, CH₃ at 2 and 6
(ix) 1-Methoxy-2-methylpropane
Structure:
CH₃
|
CH₃–CH–O–CH₃
(x) Ethoxybenzene
Common name: Phenetole
Structure: C₆H₅–O–CH₂CH₃
(xi) 1-Phenoxyheptane
Structure: C₆H₅–O–CH₂CH₂CH₂CH₂CH₂CH₂CH₃
(xii) 2-Ethoxybutane
Structure: CH₃–CH(OCH₂CH₃)–CH₂–CH₃
7.2 Write structures of the compounds whose IUPAC names are as follows:
(i) 2-Methylbutan-2-ol
Structure:
CH₃
|
CH₃–C(OH)–CH₂–CH₃
(ii) 1-Phenylpropan-2-ol
Structure: C₆H₅–CH₂–CH(OH)–CH₃
(iii) 3,5-Dimethylhexane-1,3,5-triol
Structure: CH₂OH–C(CH₃)(OH)–CH₂–C(CH₃)(OH)–CH₂OH
(iv) 2,3-Diethylphenol
Structure: Benzene ring with OH at 1, ethyl at 2 and 3
(v) 1-Ethoxypropane
Structure: CH₃–CH₂–CH₂–O–CH₂CH₃
(vi) 2-Ethoxy-3-methylpentane
Structure: CH₃–CH(OCH₂CH₃)–CH(CH₃)–CH₂–CH₃
(vii) Cyclohexylmethanol
Structure: C₆H₁₁–CH₂OH
(viii) 3-Cyclohexylpentan-3-ol
Structure: CH₃–CH₂–C(OH)(C₆H₁₁)–CH₂–CH₃
(ix) Cyclopent-3-en-1-ol
Structure: A 5-membered ring with double bond between C3 and C4 and OH on C1
(x) 4-Chloro-3-ethylbutan-1-ol
Structure: HO–CH₂–CH(Cl)–CH(C₂H₅)–CH₃
7.3 (i) Draw the structures of all isomeric alcohols of molecular formula C₅H₁₂O and give their IUPAC names.
There are 8 isomeric alcohols of C₅H₁₂O:
Pentan-1-ol
Pentan-2-ol
2-Methylbutan-1-ol
2-Methylbutan-2-ol
3-Methylbutan-1-ol
3-Methylbutan-2-ol
3-Pentanol
2,2-Dimethylpropan-1-ol
(ii) Classify the isomers as primary, secondary, or tertiary alcohols:
Primary (1°):
Pentan-1-ol
2-Methylbutan-1-ol
3-Methylbutan-1-ol
2,2-Dimethylpropan-1-ol
Secondary (2°):
Pentan-2-ol
3-Pentanol
3-Methylbutan-2-ol
Tertiary (3°):
2-Methylbutan-2-ol
7.4 Explain why propanol has a higher boiling point than that of the hydrocarbon, butane?
Answer:
Propanol (CH₃CH₂CH₂OH) contains an –OH group, capable of forming intermolecular hydrogen bonds.
Butane (C₄H₁₀) is a non-polar hydrocarbon and exhibits only weak van der Waals forces.
Hydrogen bonding in propanol requires more energy to break during boiling, hence higher boiling point.
7.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer:
Alcohols contain an –OH group, which forms hydrogen bonds with water molecules.
This allows alcohols to mix and dissolve easily in water.
Hydrocarbons are non-polar and cannot form hydrogen bonds, thus are poorly soluble in water.
Continuing with the NCERT Class 12 Chemistry Chapter “Alcohols, Phenols and Ethers” (2025–26 edition) — here are the detailed answers for Questions 7.6 to 7.12, with step-by-step explanations, correct formatting, and rechecked accuracy.
7.6 What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Answer:
Hydroboration-oxidation is a two-step reaction that converts alkenes to alcohols:
Hydroboration: Addition of BH₃ or B₂H₆ to the alkene in a syn-addition manner.
Oxidation: Treatment with H₂O₂ and NaOH converts the alkylborane to alcohol.
Example:
CH₃–CH=CH₂ (propene)
→(1. BH₃·THF
2. H₂O₂/NaOH)
→ CH₃–CH₂–CH₂OH (propan-1-ol)
Note: This reaction gives anti-Markovnikov alcohol (OH adds to less substituted carbon).
7.7 Give the structures and IUPAC names of monohydric phenols of molecular formula, C₇H₈O.
Answer:
C₇H₈O = C₆H₅–OH + CH₃ group (methyl substituted phenols)
Three isomers (cresols):
o-Cresol: 2-Methylphenol
OH at position 1, CH₃ at 2
m-Cresol: 3-Methylphenol
OH at 1, CH₃ at 3
p-Cresol: 4-Methylphenol
OH at 1, CH₃ at 4
7.8 While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Answer:
Ortho-nitrophenol is steam volatile.
Reason: It forms intramolecular hydrogen bonding, reducing its boiling point and making it volatile.
Para-nitrophenol forms intermolecular H-bonding, increasing boiling point and making it less volatile.
7.9 Give the equations of reactions for the preparation of phenol from cumene.
Answer:
Step 1: Oxidation of cumene
C₆H₅–CH(CH₃)₂ + O₂ → C₆H₅–C(CH₃)₂–OOH (cumene hydroperoxide)
Step 2: Acidic hydrolysis
Cumene hydroperoxide → C₆H₅OH (phenol) + CH₃COCH₃ (acetone)
Overall Reaction:
C₆H₅–CH(CH₃)₂ + O₂ → C₆H₅OH + CH₃COCH₃
7.10 Write chemical reaction for the preparation of phenol from chlorobenzene.
Answer:
Reagents: NaOH, high temperature and pressure
Reaction:
C₆H₅Cl + 6% NaOH →(623K, 300 atm)→ C₆H₅ONa →(H⁺)→ C₆H₅OH (phenol)
Overall:
C₆H₅Cl + NaOH → C₆H₅OH + NaCl
7.11 Write the mechanism of hydration of ethene to yield ethanol.
Answer:
Reagents: H₂SO₄ and H₂O (acidic hydration)
Mechanism:
Step 1: Protonation of ethene
CH₂=CH₂ + H⁺ → CH₃–CH₂⁺ (carbocation)
Step 2: Nucleophilic attack by water
CH₃–CH₂⁺ + H₂O → CH₃–CH₂OH₂⁺
Step 3: Deprotonation
CH₃–CH₂OH₂⁺ → CH₃–CH₂OH + H⁺
Product: Ethanol (CH₃CH₂OH)
7.12 You are given benzene, conc. H₂SO₄ and NaOH. Write the equations for the preparation of phenol using these reagents.
Answer:
Step 1: Sulphonation of benzene
C₆H₆ + conc. H₂SO₄ → C₆H₅SO₃H (benzene sulphonic acid)
Step 2: Fusion with NaOH
C₆H₅SO₃H + 2NaOH → C₆H₅ONa + Na₂SO₃ + H₂O
Step 3: Acidification
C₆H₅ONa + HCl → C₆H₅OH (phenol) + NaCl
7.13 Show how will you synthesise:
(i) 1-Phenylethanol from a suitable alkene
Answer: Use styrene (C₆H₅CH=CH₂) and hydroboration-oxidation
Step 1: C₆H₅CH=CH₂ → (1. BH₃·THF, 2. H₂O₂/NaOH) → C₆H₅CH(OH)CH₃
Product: 1-Phenylethanol
Reaction follows anti-Markovnikov rule
(ii) Cyclohexylmethanol using an alkyl halide by SN2 reaction
Answer:
Reagent: Cyclohexylmethyl bromide (C₆H₁₁CH₂Br)
React with aqueous NaOH:
C₆H₁₁CH₂Br + OH⁻ → C₆H₁₁CH₂OH + Br⁻
Mechanism: SN2 substitution
(iii) Pentan-1-ol using a suitable alkyl halide
Answer:
Reagent: 1-bromopentane
React with NaOH:
CH₃–CH₂–CH₂–CH₂–CH₂Br + OH⁻ → CH₃–CH₂–CH₂–CH₂–CH₂OH
Mechanism: SN2 reaction
7.14 Give two reactions that show the acidic nature of phenol. Compare acidity of phenol with that of ethanol.
Answer:
(i) Reaction with NaOH:
C₆H₅OH + NaOH → C₆H₅O⁻Na⁺ + H₂O
(ii) Reaction with Na metal:
2C₆H₅OH + 2Na → 2C₆H₅ONa + H₂↑
Comparison with ethanol:
Phenol is more acidic than ethanol due to resonance stabilization of phenoxide ion.
In ethanol, the ethoxide ion is not resonance-stabilized, making ethanol a weaker acid.
7.15 Explain why ortho nitrophenol is more acidic than ortho methoxyphenol.
Answer:
Ortho-nitrophenol: Electron-withdrawing –NO₂ group stabilizes the phenoxide ion via –M (mesomeric) and –I (inductive) effects → increases acidity.
Ortho-methoxyphenol: –OCH₃ group is electron-donating by +M effect → destabilizes phenoxide ion → decreases acidity.
Hence, o-nitrophenol > o-methoxyphenol in acidity.
7.16 Explain how the –OH group attached to a carbon of benzene ring activates it towards electrophilic substitution.
Answer:
The –OH group donates electron density via +M (resonance) effect.
It increases electron density particularly at the ortho and para positions.
Therefore, it activates the benzene ring toward electrophilic attack.
7.17 Give equations of the following reactions:
(i) Oxidation of propan-1-ol with alkaline KMnO₄
CH₃CH₂CH₂OH →(alkaline KMnO₄)→ CH₃CH₂COOH
(ii) Bromine in CS₂ with phenol
C₆H₅OH + 3Br₂ → C₆H₂Br₃OH + 3HBr
(Product: 2,4,6-tribromophenol)
(iii) Dilute HNO₃ with phenol
C₆H₅OH + HNO₃ → o-nitrophenol + p-nitrophenol + H₂O
(iv) Treating phenol with chloroform in presence of aqueous NaOH (Reimer-Tiemann Reaction)
C₆H₅OH + CHCl₃ + 3NaOH → o-Hydroxybenzaldehyde + 3NaCl + 2H₂O
7.18 Explain the following with an example:
(i) Kolbe’s Reaction:
Phenol reacts with NaOH and CO₂ at 400 K and 6 atm
C₆H₅ONa + CO₂ → o-Hydroxybenzoic acid (salicylic acid)
(ii) Reimer-Tiemann Reaction:
Phenol + CHCl₃ + NaOH → o-Hydroxybenzaldehyde (salicylaldehyde)
(iii) Williamson Ether Synthesis:
RX + R’O⁻ → ROR’
Example: CH₃CH₂Br + NaOCH₃ → CH₃CH₂OCH₃ + NaBr
(iv) Unsymmetrical ether:
Ethers with two different alkyl/aryl groups
Example: CH₃OCH₂CH₃ (methoxyethane)
7.19 Write the mechanism of acid dehydration of ethanol to yield ethene.
Mechanism (E1):
Step 1: Protonation
CH₃CH₂OH + H⁺ → CH₃CH₂OH₂⁺
Step 2: Formation of carbocation
CH₃CH₂OH₂⁺ → CH₃CH₂⁺ + H₂O
Step 3: Elimination
CH₃CH₂⁺ → CH₂=CH₂ + H⁺
Product: Ethene + H₂O
Catalyst: Conc. H₂SO₄, 443 K
7.20 How are the following conversions carried out?
(i) Propene → Propan-2-ol
CH₃–CH=CH₂ →(H₂O/H⁺)→ CH₃–CHOH–CH₃
(Via acid-catalysed hydration, Markovnikov addition)
(ii) Benzyl chloride → Benzyl alcohol
C₆H₅CH₂Cl + NaOH → C₆H₅CH₂OH + NaCl
(iii) Ethyl magnesium chloride → Propan-1-ol
CH₃CH₂MgCl + HCHO → CH₃CH₂CH₂OMgCl →(H⁺/H₂O)→ CH₃CH₂CH₂OH
(iv) Methyl magnesium bromide → 2-Methylpropan-2-ol
(CH₃)MgBr + CH₃COCH₃ → (CH₃)₃COMgBr →(H⁺/H₂O)→ (CH₃)₃COH
7.21 Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid
Reagent: Acidified potassium permanganate (KMnO₄) or Jones reagent (CrO₃ in H₂SO₄)
(ii) Oxidation of a primary alcohol to aldehyde
Reagent: Pyridinium chlorochromate (PCC) or Dess–Martin periodinane
(iii) Bromination of phenol to 2,4,6-tribromophenol
Reagent: Br₂ in water
(iv) Benzyl alcohol to benzoic acid
Reagent: Acidified KMnO₄
(v) Dehydration of propan-2-ol to propene
Reagent: Conc. H₂SO₄, 443 K
(vi) Butan-2-one to butan-2-ol
Reagent: NaBH₄ or LiAlH₄ (reducing agents)
7.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer:
Ethanol (CH₃CH₂OH) forms strong intermolecular hydrogen bonds due to –OH group.
Methoxymethane (CH₃OCH₃), an ether, lacks –OH group and cannot form H-bonds.
Therefore, ethanol has a higher boiling point.
7.23 Give IUPAC names of the following ethers:
(i) Dimethyl ether → Methoxy methane
(ii) Diethyl ether → Ethoxy ethane
(iii) Methyl propyl ether → 1-Methoxypropane
(iv) Anisole → Methoxybenzene
(v) Phenetole → Ethoxybenzene
(vi) Heptyl phenyl ether → 1-Phenoxyheptane
7.24 Write the names of reagents and equations for the preparation of the following ethers by Williamson’s synthesis:
(i) 1-Propoxypropane
Reagents: Propan-1-ol, Sodium, 1-bromopropane
CH₃CH₂CH₂ONa + CH₃CH₂CH₂Br → CH₃CH₂CH₂OCH₂CH₂CH₃
(ii) Ethoxybenzene
Reagents: Phenol, NaOH, Ethyl bromide
C₆H₅ONa + C₂H₅Br → C₆H₅OC₂H₅ + NaBr
(iii) 2-Methoxy-2-methylpropane
Reagents: Sodium methoxide and tert-butyl bromide
CH₃O⁻Na⁺ + (CH₃)₃CBr → (CH₃)₃COCH₃ + NaBr
(iv) 1-Methoxyethane
Reagents: Sodium ethoxide and methyl bromide
CH₃CH₂ONa + CH₃Br → CH₃CH₂OCH₃ + NaBr
7.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
Answer:
Limitation: When a tertiary alkyl halide is used, elimination (E2) competes with substitution.
Example:
(CH₃)₃CBr + NaOC₂H₅ → (CH₃)₂C=CH₂ (alkene, via elimination)
Better: Use tertiary alcohol to make alkoxide and react with a primary halide instead.
7.26 How is 1-propoxypropane synthesised from propan-1-ol? Write mechanism of this reaction.
Answer:
Step 1: Convert propan-1-ol to sodium propoxide
CH₃CH₂CH₂OH + Na → CH₃CH₂CH₂ONa + ½H₂
Step 2: React with 1-bromopropane
CH₃CH₂CH₂ONa + CH₃CH₂CH₂Br → CH₃CH₂CH₂OCH₂CH₂CH₃ + NaBr
Mechanism:
SN2 substitution
Nucleophile (alkoxide) attacks primary carbon of alkyl halide → ether
7.27 Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Answer:
Secondary and tertiary alcohols form carbocations under acidic dehydration conditions.
These carbocations are prone to elimination → alkene formation, rather than ether.
Example:
(CH₃)₃COH → (CH₃)₂C=CH₂ (alkene)
Therefore, elimination dominates, making this method unsuitable for 2° and 3° alcohols.
Here is the final set of answers for NCERT Class 12 Chemistry Chapter “Alcohols, Phenols and Ethers” (2025–26 edition) — detailed, step-by-step solutions for Questions 7.28 to 7.33, all rechecked for correctness and rendering.
7.28 Write the equation of the reaction of hydrogen iodide with:
(i) 1-propoxypropane
CH₃CH₂CH₂–O–CH₂CH₂CH₃ + HI → CH₃CH₂CH₂OH + CH₃CH₂CH₂I
(ii) Methoxybenzene (anisole)
C₆H₅–O–CH₃ + HI → C₆H₅OH + CH₃I
(Phenol is not further cleaved)
(iii) Benzyl ethyl ether
C₆H₅CH₂–O–CH₂CH₃ + HI → C₆H₅CH₂OH + C₂H₅I
7.29 Explain the fact that in aryl alkyl ethers:
(i) The alkoxy group activates the benzene ring towards electrophilic substitution
→ The –OR group shows +M (resonance) effect, donating electrons to the benzene ring → increases reactivity.
(ii) It directs the incoming substituents to ortho and para positions
→ Due to resonance, electron density increases at ortho and para positions, making them preferred sites for substitution.
7.30 Write the mechanism of the reaction of HI with methoxymethane.
Answer:
Step 1: Protonation of ether
CH₃–O–CH₃ + H⁺ → CH₃–OH⁺–CH₃
Step 2: Nucleophilic attack by I⁻
CH₃–OH⁺–CH₃ + I⁻ → CH₃OH + CH₃I
Product: Methanol + Methyl iodide
Mechanism type: SN2
7.31 Write equations of the following reactions:
(i) Friedel–Crafts alkylation of anisole
C₆H₅OCH₃ + CH₃Cl + AlCl₃ → o- and p-methylanisole
(ii) Nitration of anisole
C₆H₅OCH₃ + HNO₃ + H₂SO₄ → o- and p-nitroanisole
(iii) Bromination of anisole in ethanoic acid medium
C₆H₅OCH₃ + Br₂ (in CH₃COOH) → o- and p-bromoanisole
(iv) Friedel–Crafts acetylation of anisole
C₆H₅OCH₃ + CH₃COCl + AlCl₃ → o- and p-acetylanisole
7.32 Show how would you synthesise the following alcohols from appropriate alkenes:
(i) Methanol
CO + 2H₂ → CH₃OH (catalytic hydrogenation)
(ii) Primary alcohol structures
E.g. CH₂=CH₂ + BH₃, then H₂O₂/NaOH → CH₃CH₂OH (ethanol)
(iii) Secondary alcohol structures
E.g. CH₃–CH=CH₂ + H₂O (acidic hydration) → CH₃–CHOH–CH₃ (propan-2-ol)
(iv) Tertiary alcohol structures
E.g. (CH₃)₂C=CH₂ + H₂O/H⁺ → (CH₃)₃COH (tert-butanol)
7.33 When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place. Give a mechanism for this reaction.
Answer:
Compound:
CH₃–CH(CH₃)–CH(OH)–CH₃
Step-by-step mechanism:
Step 1: Protonation of –OH
Alcohol + HBr → R–OH₂⁺
Step 2: Formation of secondary carbocation
Loss of water → 2° carbocation (CH₃–CH(CH₃)–CH⁺–CH₃)
Step 3: Hydride shift from adjacent carbon
3° carbocation forms via hydride shift: CH₃–C⁺(CH₃)–CH₂–CH₃
Step 4: Nucleophilic attack by Br⁻
Final product: 2-bromo-2-methylbutane
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
(CBSE MODEL QUESTIONS PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
Q1. Which of the following is the most acidic compound?
(A) Ethanol
(B) Cyclohexanol
(C) Water
(D) Phenol
Answer: (D) Phenol
Q2. The IUPAC name of CH₃CH₂OCH₂CH₃ is:
(A) Methoxyethane
(B) Diethyl ether
(C) Ethoxyethane
(D) Ethyl methyl ether
Answer: (C) Ethoxyethane
Q3. Lucas test is used to distinguish:
(A) Aldehydes
(B) Primary, secondary, and tertiary alcohols
(C) Ethers
(D) Phenols
Answer: (B) Primary, secondary, and tertiary alcohols
Q4. Which one of the following alcohols cannot be prepared by the reduction of a carbonyl compound?
(A) Methanol
(B) Ethanol
(C) Tert-butanol
(D) Isopropanol
Answer: (C) Tert-butanol
Q5. Which alcohol gives turbidity immediately in Lucas test?
(A) 1° alcohol
(B) 2° alcohol
(C) 3° alcohol
(D) Phenol
Answer: (C) 3° alcohol
Q6. Assertion (A): Phenol is more acidic than ethanol.
Reason (R): Phenoxide ion is resonance stabilized.
(A) Both A and R are true, and R is the correct explanation of A
(B) Both A and R are true, but R is not the correct explanation of A
(C) A is true, R is false
(D) A is false, R is true
Answer: (A) Both A and R are true, and R is the correct explanation of A
Q7. Assertion (A): Ethers have lower boiling points than alcohols.
Reason (R): Ethers do not form intermolecular hydrogen bonding.
(A) Both A and R are true, and R is the correct explanation of A
(B) Both A and R are true, but R is not the correct explanation of A
(C) A is true, R is false
(D) A is false, R is true
Answer: (A) Both A and R are true, and R is the correct explanation of A
Q8. Which of the following undergoes electrophilic substitution more easily?
(A) Benzene
(B) Toluene
(C) Phenol
(D) Aniline
Answer: (C) Phenol
Q9. The product formed when phenol is treated with bromine water is:
(A) o-Bromophenol
(B) p-Bromophenol
(C) 2,4,6-Tribromophenol
(D) 3-Bromophenol
Answer: (C) 2,4,6-Tribromophenol
Q10. The IUPAC name of CH₃CH(OH)CH₃ is:
(A) Propan-2-ol
(B) Isopropyl alcohol
(C) Propan-1-ol
(D) Ethyl methyl alcohol
Answer: (A) Propan-2-ol
Q11. Case-Based MCQ:
Read the passage and answer the question.
Phenol undergoes electrophilic substitution reactions more readily than benzene due to the electron-donating effect of the hydroxyl group, activating the ortho and para positions.
Which of the following reactions supports this property?
(A) Friedel–Crafts alkylation
(B) Bromination of phenol forming 2,4,6-tribromophenol
(C) Reduction of phenol
(D) Nucleophilic substitution
Answer: (B) Bromination of phenol forming 2,4,6-tribromophenol
Q12. Case-Based MCQ:
Ethanol is widely used as a biofuel and industrial solvent. It is miscible with water and has antiseptic properties. Its boiling point is significantly higher than that of dimethyl ether due to hydrogen bonding.
Which of the following is NOT true about ethanol?
(A) It is a good antiseptic
(B) It forms strong intermolecular hydrogen bonds
(C) It is less polar than dimethyl ether
(D) It is used as fuel
Answer: (C) It is less polar than dimethyl ether
Q13. Which among the following undergoes Kolbe’s reaction?
(A) Methanol
(B) Benzene
(C) Phenol
(D) Anisole
Answer: (C) Phenol
Q14. Williamson ether synthesis is NOT suitable for:
(A) Methyl iodide + Sodium ethoxide
(B) Ethyl bromide + Sodium methoxide
(C) Tert-butyl bromide + Sodium ethoxide
(D) Benzyl bromide + Sodium phenoxide
Answer: (C) Tert-butyl bromide + Sodium ethoxide
Q15. Which of the following will give a violet colour with neutral FeCl₃?
(A) Benzene
(B) Phenol
(C) Ethanol
(D) Toluene
Answer: (B) Phenol
Q16. The major product of dehydration of ethanol at 443 K in presence of concentrated H₂SO₄ is:
(A) Diethyl ether
(B) Ethene
(C) Acetaldehyde
(D) Methane
Answer: (B) Ethene
Q17. Which of the following does not react with Na metal to liberate hydrogen?
(A) Ethanol
(B) Phenol
(C) Water
(D) Diethyl ether
Answer: (D) Diethyl ether
Q18. The role of dry HCl in the preparation of ethers is to:
(A) Protonate the ether
(B) Catalyse the reaction
(C) Remove water
(D) Oxidise the alcohol
Answer: (B) Catalyse the reaction
🔹 SECTION B (2 Marks Each)
Q19. Write the mechanism of acid-catalysed hydration of ethene to form ethanol.
Answer:
Step 1: Protonation of ethene → formation of carbocation
CH₂=CH₂ + H⁺ → CH₃−CH₂⁺
Step 2: Nucleophilic attack by water
CH₃−CH₂⁺ + H₂O → CH₃−CH₂OH₂⁺
Step 3: Deprotonation
CH₃−CH₂OH₂⁺ → CH₃−CH₂OH + H⁺
Q20. Why are phenols more acidic than alcohols?
Answer:
Phenols form phenoxide ion after losing H⁺, which is resonance stabilized. Alcohols form alkoxide ions, which are not resonance stabilized. The stability of phenoxide ion increases the acidity of phenol.
Q21. How will you distinguish between 1°, 2°, and 3° alcohols using Lucas reagent?
Answer:
Lucas reagent: ZnCl₂ + conc. HCl
3° alcohol → immediate turbidity
2° alcohol → turbidity in 5–10 mins
1° alcohol → no turbidity at room temp
Q22. Write the equation for the preparation of phenol from cumene.
Answer:
Step 1: Air oxidation of cumene
C₆H₅CH(CH₃)₂ + O₂ → C₆H₅C(CH₃)(OOH)
Step 2: Acidic hydrolysis
C₆H₅C(CH₃)(OOH) + H⁺ → C₆H₅OH + CH₃COCH₃
Q23. An organic compound A with molecular formula C₄H₁₀O gives Lucas test immediately. On oxidation, it gives compound B with molecular formula C₄H₈O. Identify A and B and give their IUPAC names.
Answer:
Compound A is tert-butyl alcohol (2-methylpropan-2-ol).
Compound B is 2-methylpropanone (a ketone).
A: (CH₃)₃COH → B: (CH₃)₂CO
🔹 SECTION C (3 Marks Each)
Q24. Write the steps involved in the conversion of phenol to salicylic acid using Kolbe’s reaction. Give the equation.
Answer:
Step 1: Phenol reacts with NaOH to form phenoxide ion
C₆H₅OH + NaOH → C₆H₅O⁻Na⁺ + H₂O
Step 2: Phenoxide reacts with CO₂ (at 400 K, 6 atm)
C₆H₅O⁻Na⁺ + CO₂ → o-HOC₆H₄COONa
Step 3: Acidification
o-HOC₆H₄COONa + HCl → o-HOC₆H₄COOH (salicylic acid)
Q25. Explain Williamson’s ether synthesis with a suitable example. Why is it not suitable for aryl halides?
Answer:
Example:
C₂H₅ONa + CH₃Br → CH₃OC₂H₅ + NaBr
This is a nucleophilic substitution (SN2) reaction.
Not suitable for aryl halides like bromobenzene because aryl halides do not undergo SN2 reaction due to partial double bond character in C–Br bond.
Q26. Describe the mechanism of dehydration of ethanol to form ethene. Mention the catalyst and temperature.
Answer:
Catalyst: Conc. H₂SO₄
Temp: 443 K
Step 1: Protonation of −OH group
CH₃CH₂OH + H⁺ → CH₃CH₂OH₂⁺
Step 2: Loss of water
CH₃CH₂OH₂⁺ → CH₃CH₂⁺ + H₂O
Step 3: Formation of alkene
CH₃CH₂⁺ → CH₂=CH₂ + H⁺
Q27. How will you convert the following:
(a) Phenol to Anisole
(b) Ethanol to Ethene
(c) Ethene to Ethanol
Answer:
(a) C₆H₅OH + NaOH → C₆H₅O⁻Na⁺ → + CH₃I → C₆H₅OCH₃
(b) CH₃CH₂OH →(conc. H₂SO₄, 443 K)→ CH₂=CH₂ + H₂O
(c) CH₂=CH₂ + H₂O →(H⁺ catalyst)→ CH₃CH₂OH
Q28. A compound C₇H₈O on treatment with bromine water gives a white precipitate. On heating with Zn dust, it gives toluene. Identify the compound and write reactions involved.
Answer:
The compound is o-cresol or p-cresol (methylphenol).
C₆H₄(CH₃)(OH) + 3Br₂ → C₆H₂Br₃(CH₃)(OH) + 3HBr
C₆H₄(CH₃)(OH) + Zn → C₆H₅CH₃ + ZnO
🔹 SECTION D (4 Marks Each) – Case-Based Questions
Q29. Read the passage below and answer the following:
An organic compound (A) with molecular formula C₂H₆O is found to be soluble in water and gives effervescence with sodium. It undergoes oxidation with acidified K₂Cr₂O₇ to give compound (B), which further oxidizes to compound (C), a carboxylic acid.
(a) Identify A, B, and C.
(b) Write balanced equations for both oxidation steps.
(c) What functional group test would confirm compound (C)?
Answer:
(a)
A = Ethanol (CH₃CH₂OH)
B = Ethanal (CH₃CHO)
C = Ethanoic acid (CH₃COOH)
(b)
CH₃CH₂OH + [O] → CH₃CHO + H₂O
CH₃CHO + [O] → CH₃COOH
(c)
Test for carboxylic acid:
NaHCO₃ test → Effervescence due to CO₂
Q30. Read the passage and answer:
Phenol is known for undergoing electrophilic substitution reactions readily. On treatment with bromine water, it gives a white precipitate. It also gives coloured complex with FeCl₃.
(a) Why does phenol undergo these reactions more readily than benzene?
(b) Write the equation for bromination of phenol.
(c) What is the product of Reimer–Tiemann reaction? Write the equation.
Answer:
(a) The −OH group activates the aromatic ring by resonance and increases electron density at ortho and para positions.
(b)
C₆H₅OH + 3Br₂ → C₆H₂Br₃OH + 3HBr (white ppt of 2,4,6-tribromophenol)
(c) Reimer–Tiemann reaction:
C₆H₅OH + CHCl₃ + 3NaOH → o-HO−C₆H₄−CHO + 3NaCl + 2H₂O
Q31. Case study:
An organic chemist is trying to prepare an ether using Williamson synthesis. He reacts sodium ethoxide with 2-bromo-2-methylpropane.
(a) Will the reaction proceed? Why or why not?
(b) Suggest a better pair of reactants for synthesising ethyl tert-butyl ether.
(c) Write the reaction.
Answer:
(a) No, the reaction is not feasible. Tertiary alkyl halides undergo elimination in presence of strong bases like ethoxide.
(b) Use sodium tert-butoxide and ethyl bromide.
(c)
(CH₃)₃CO⁻Na⁺ + CH₃CH₂Br → (CH₃)₃COCH₂CH₃ + NaBr
🔹 SECTION E (5 Marks Each) – Long Answer Questions
Q32. Write a comparative account of acidity of alcohols, phenols, and water. Include explanation using resonance, inductive effect, and examples.
Answer:
Alcohols: Weakly acidic. On loss of H⁺, the resulting alkoxide ion is not resonance-stabilized. Inductive effect of alkyl group destabilizes the ion.
Water: Slightly more acidic than alcohol due to absence of electron-releasing groups.
Phenols: Most acidic among the three. Phenoxide ion is resonance stabilized. The −OH group donates electron density into aromatic ring, delocalizing the negative charge.
Order of acidity: Phenol > Water > Alcohol
Example reactions with Na:
Phenol + Na → Phenoxide + ½ H₂
Alcohol + Na → Alkoxide + ½ H₂
Phenol gives colour with FeCl₃, showing stronger acidic behaviour.
Q33. Explain the mechanism of Williamson ether synthesis. Illustrate the limitations using examples. Why are aryl halides not suitable in this synthesis?
Answer:
Mechanism:
RO⁻ + R′−X → R−O−R′ + X⁻ (SN2 mechanism)
Nucleophile = Alkoxide ion
Electrophile = Alkyl halide
Preferred: 1° alkyl halide due to less steric hindrance
Limitations:
3° alkyl halides undergo elimination (E2) instead of substitution
Aryl halides (e.g., bromobenzene) do not react due to resonance stabilization of C−Br bond and inability to undergo backside attack
Example (Good):
CH₃ONa + C₂H₅Br → CH₃OC₂H₅ + NaBr
Example (Bad):
(CH₃)₃CBr + CH₃ONa → Elimination to alkene, not ether
Q34. Compare and explain the reactions of ethanol, phenol, and ether with the following:
(a) Sodium metal
(b) Acidic dehydration
(c) Reaction with HX
Answer:
(a) Sodium metal
Ethanol and phenol react with Na, evolve H₂
Ethers do not react
C₂H₅OH + Na → C₂H₅ONa + ½H₂
C₆H₅OH + Na → C₆H₅ONa + ½H₂
(b) Acidic dehydration
Ethanol: Forms ethene at 443 K with conc. H₂SO₄
CH₃CH₂OH → CH₂=CH₂ + H₂O
Phenol: Does not dehydrate
Ethers: Formed by dehydration of alcohols at lower temp (~413 K)
(c) Reaction with HX
Alcohols form alkyl halides
CH₃CH₂OH + HBr → CH₃CH₂Br + H₂O
Phenol: Difficult, gives aryl halides only under extreme conditions
Ethers: Cleaved by HX
CH₃OC₂H₅ + HI → CH₃I + C₂H₅OH
Q35. Write chemical tests to distinguish between the following pairs. Also give chemical equations.
(a) Ethanol and Phenol
(b) Methanol and Ethanoic acid
(c) Anisole and Phenol
Answer:
(a) Ferric chloride test:
Phenol gives violet colour
C₆H₅OH + FeCl₃ → Violet complex
Ethanol: No colour change
(b) NaHCO₃ test:
Ethanoic acid gives effervescence (CO₂)
CH₃COOH + NaHCO₃ → CH₃COONa + CO₂ + H₂O
Methanol: No reaction
(c) Bromine water:
Phenol → white ppt of 2,4,6-tribromophenol
C₆H₅OH + 3Br₂ → C₆H₂Br₃OH + 3HBr
Anisole: No reaction at room temperature
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
Q1. Which of the following compounds will give a white precipitate with bromine water?
(A) Aniline
(B) Benzene
(C) Phenol
(D) Toluene
Answer: (C) Phenol
Year: 2025 | Set: Z
Q2. The Lucas test is used to distinguish between:
(A) Primary, secondary and tertiary alcohols
(B) Alcohols and phenols
(C) Alcohols and aldehydes
(D) Alcohols and ethers
Answer: (A) Primary, secondary and tertiary alcohols
Year: 2025 | Set: 2
Q3. Which of the following is not true about phenol?
(A) It is more acidic than ethanol
(B) It undergoes electrophilic substitution easily
(C) It does not react with sodium
(D) It gives a violet colour with neutral FeCl₃
Answer: (C) It does not react with sodium
Year: 2024 | Set: Z
Q4. An organic compound C₇H₈O does not give effervescence with NaHCO₃ but forms a violet complex with FeCl₃. The compound is:
(A) Benzoic acid
(B) Benzyl alcohol
(C) o-Cresol
(D) Benzaldehyde
Answer: (C) o-Cresol
Year: 2024 | Set: 1
Q5. The acidic strength decreases in the order:
(A) Phenol > p-nitrophenol > p-methylphenol
(B) p-nitrophenol > phenol > p-methylphenol
(C) p-methylphenol > phenol > p-nitrophenol
(D) Phenol > p-methylphenol > p-nitrophenol
Answer: (B) p-nitrophenol > phenol > p-methylphenol
Year: 2023 | Set: Z
Q6. The compound that reacts with Lucas reagent fastest is:
(A) Butan-1-ol
(B) Butan-2-ol
(C) 2-Methylpropan-2-ol
(D) Ethanol
Answer: (C) 2-Methylpropan-2-ol
Year: 2023 | Set: 2
Q7. Which compound forms anisole when reacted with methyl iodide in the presence of NaOH?
(A) Benzene
(B) Phenol
(C) Toluene
(D) Benzyl alcohol
Answer: (B) Phenol
Year: 2022 | Set: M
Q8. On treatment with bromine water, phenol forms:
(A) Bromobenzene
(B) 2,4,6-Tribromophenol
(C) Benzyl bromide
(D) o-Bromophenol
Answer: (B) 2,4,6-Tribromophenol
Year: 2022 | Set: 1
Q9. Which among the following does not give violet colour with neutral FeCl₃?
(A) Phenol
(B) o-Cresol
(C) Salicylic acid
(D) Benzyl alcohol
Answer: (D) Benzyl alcohol
Year: 2021 | Set: Z
Q10. In Williamson synthesis, which combination will give diethyl ether?
(A) CH₃CH₂ONa + CH₃Br
(B) CH₃ONa + C₂H₅Br
(C) C₂H₅ONa + C₂H₅Br
(D) CH₃CH₂OH + CH₃OH
Answer: (C) C₂H₅ONa + C₂H₅Br
Year: 2021 | Set: 2
Q11. Phenol is more acidic than alcohols due to:
(A) Higher molecular weight
(B) Resonance stabilization of phenoxide ion
(C) Presence of aromatic ring
(D) Hydrogen bonding
Answer: (B) Resonance stabilization of phenoxide ion
Year: 2020 | Set: R
Q12. Dehydration of alcohols to alkenes is catalysed by:
(A) H₂O
(B) Conc. HCl
(C) Conc. H₂SO₄
(D) Dil. NaOH
Answer: (C) Conc. H₂SO₄
Year: 2020 | Set: 3
Q13. The major product formed when phenol reacts with CHCl₃ and NaOH followed by acidification is:
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) o-Hydroxybenzaldehyde
(D) p-Hydroxybenzaldehyde
Answer: (C) o-Hydroxybenzaldehyde
Year: 2019 | Set: Q
Q14. In the Kolbe reaction, the electrophile is:
(A) CO₂
(B) CHCl₃
(C) Cl⁺
(D) Br⁺
Answer: (A) CO₂
Year: 2019 | Set: 2
Q15. An ether is more volatile than its isomeric alcohol because:
(A) It forms hydrogen bond
(B) It is non-polar
(C) It has lower molecular weight
(D) It does not form hydrogen bond
Answer: (D) It does not form hydrogen bond
Year: 2018 | Set: S
Q16. Which of the following is an example of a primary alcohol?
(A) Isopropanol
(B) tert-Butanol
(C) Ethanol
(D) 2-Butanol
Answer: (C) Ethanol
Year: 2018 | Set: 3
Q17. The major product formed when ethanol is heated with conc. H₂SO₄ at 443 K is:
(A) Acetaldehyde
(B) Ethene
(C) Diethyl ether
(D) Acetic acid
Answer: (B) Ethene
Year: 2017 | Set: Z
Q18. When phenol is treated with dilute nitric acid at 298 K, the major product is:
(A) o-Nitrophenol
(B) m-Nitrophenol
(C) p-Nitrophenol
(D) 2,4,6-Trinitrophenol
Answer: (A) o-Nitrophenol
Year: 2017 | Set: 2
Q19. Which of the following compounds will give only one monosubstituted product on reaction with Br₂/FeBr₃?
(A) Phenol
(B) Aniline
(C) Benzene
(D) p-Nitrophenol
Answer: (D) p-Nitrophenol
Year: 2016 | Set: N
Q20. Which of the following ethers can be prepared by Williamson synthesis?
(A) tert-Butyl methyl ether
(B) Benzyl methyl ether
(C) Phenyl methyl ether
(D) Diphenyl ether
Answer: (B) Benzyl methyl ether
Year: 2016 | Set: 3
Q21. The reagent used to oxidise ethanol to acetic acid is:
(A) Zn/HCl
(B) H₂/Ni
(C) Acidified K₂Cr₂O₇
(D) Na
Answer: (C) Acidified K₂Cr₂O₇
Year: 2015 | Set: L
Q22. Which of the following alcohols will react fastest with HCl/ZnCl₂?
(A) 1-Propanol
(B) 2-Propanol
(C) 2-Methyl-2-propanol
(D) Ethanol
Answer: (C) 2-Methyl-2-propanol
Year: 2015 | Set: 1
Q23. Reimer–Tiemann reaction involves:
(A) Chlorination
(B) Carboxylation
(C) Formylation
(D) Sulphonation
Answer: (C) Formylation
Year: 2014 | Set: S
Q24. Phenol is a weak acid. It dissolves in NaOH forming:
(A) Sodium phenyl
(B) Sodium phenoxide
(C) Phenolate
(D) Both B and C
Answer: (D) Both B and C
Year: 2014 | Set: 3
Q25. In which of the following, the −OH group is most acidic?
(A) CH₃OH
(B) Phenol
(C) o-Nitrophenol
(D) p-Methoxyphenol
Answer: (C) o-Nitrophenol
Year: 2013 | Set: M
Q26. What happens when phenol is treated with bromine water?
(A) Forms bromobenzene
(B) Undergoes substitution at meta position
(C) Forms 2,4,6-tribromophenol
(D) No reaction
Answer: (C) Forms 2,4,6-tribromophenol
Year: 2013 | Set: 2
Q27. Which of the following will give a clear solution with NaOH but no effervescence with NaHCO₃?
(A) Benzoic acid
(B) Phenol
(C) Ethanoic acid
(D) Salicylic acid
Answer: (B) Phenol
Year: 2012 | Set: O
Q28. The major product of dehydration of ethanol using conc. H₂SO₄ at 413 K is:
(A) Ethene
(B) Diethyl ether
(C) Acetaldehyde
(D) Acetic acid
Answer: (B) Diethyl ether
Year: 2012 | Set: 1
Q29. Which one gives violet coloration with FeCl₃ solution?
(A) Phenol
(B) Benzyl alcohol
(C) Acetone
(D) Cyclohexanol
Answer: (A) Phenol
Year: 2011 | Set: Q
Q30. On oxidation, ethanol gives:
(A) Acetic acid
(B) Acetone
(C) Methanoic acid
(D) Propanoic acid
Answer: (A) Acetic acid
Year: 2011 | Set: 2
Q31. The major product formed when sodium phenoxide reacts with CO₂ under pressure and then acidified is:
(A) Benzoic acid
(B) Salicylic acid
(C) o-Hydroxybenzaldehyde
(D) Anisole
Answer: (B) Salicylic acid
Year: 2010 | Set: S
Q32. Diethyl ether is prepared from ethanol using:
(A) NaOH
(B) H₂SO₄ (443 K)
(C) H₂SO₄ (413 K)
(D) HCl
Answer: (C) H₂SO₄ (413 K)
Year: 2010 | Set: 1
Q33. Which one will not undergo SN2 reaction in Williamson synthesis?
(A) 1-Bromopropane
(B) Benzyl bromide
(C) tert-Butyl bromide
(D) Methyl iodide
Answer: (C) tert-Butyl bromide
Year: 2009 | Set: M
Q34. The electrophile involved in the Reimer–Tiemann reaction is:
(A) CO₂
(B) Cl⁺
(C) CHO⁺
(D) Dichlorocarbene (:CCl₂)
Answer: (D) Dichlorocarbene (:CCl₂)
Year: 2009 | Set: 2
Q35. The best combination for Williamson synthesis of ethoxybenzene is:
(A) C₂H₅OH and C₆H₅Cl
(B) C₂H₅Br and C₆H₅ONa
(C) C₆H₅Br and C₂H₅ONa
(D) C₂H₅OH and C₆H₅ONa
Answer: (B) C₂H₅Br and C₆H₅ONa
Year: 2008 | Set: M
Q36. Reimer–Tiemann reaction of phenol with CHCl₃ and NaOH gives:
(A) o-Hydroxybenzaldehyde
(B) p-Hydroxybenzaldehyde
(C) Salicylic acid
(D) Benzaldehyde
Answer: (A) o-Hydroxybenzaldehyde
Year: 2008 | Set: 1
Q37. Which of the following alcohols will not give Lucas test?
(A) tert-Butyl alcohol
(B) sec-Butyl alcohol
(C) Ethanol
(D) Isopropyl alcohol
Answer: (C) Ethanol
Year: 2007 | Set: Q
Q38. What is formed when phenol is treated with chloroform and alcoholic KOH?
(A) Benzaldehyde
(B) o-Hydroxybenzaldehyde
(C) o-Hydroxyacetophenone
(D) Benzoic acid
Answer: (B) o-Hydroxybenzaldehyde
Year: 2007 | Set: 2
Q39. Which of the following reacts with Na to give hydrogen gas?
(A) Diethyl ether
(B) Phenol
(C) Benzene
(D) Anisole
Answer: (B) Phenol
Year: 2006 | Set: Z
Q40. Which among the following has the lowest boiling point?
(A) Water
(B) Ethanol
(C) Phenol
(D) Dimethyl ether
Answer: (D) Dimethyl ether
Year: 2006 | Set: 1
Q41. Alcohols are generally prepared in the laboratory by:
(A) Hydrolysis of alkenes
(B) Dehydration of ethers
(C) Oxidation of acids
(D) Dehydrohalogenation of alkyl halides
Answer: (A) Hydrolysis of alkenes
Year: 2005 | Set: M
Q42. The product formed by reaction of phenol with NaOH followed by CO₂ is:
(A) Salicylic acid
(B) Phenoxide
(C) p-Hydroxybenzoic acid
(D) o-Hydroxybenzaldehyde
Answer: (A) Salicylic acid
Year: 2005 | Set: 2
Q43. Which reagent is used to convert alcohol to aldehyde?
(A) Conc. H₂SO₄
(B) Alkaline KMnO₄
(C) PCC (Pyridinium chlorochromate)
(D) NaBH₄
Answer: (C) PCC
Year: 2004 | Set: N
Q44. Which of the following is not formed when phenol is heated with concentrated nitric acid?
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) 2,4,6-Trinitrophenol
(D) m-Nitrophenol
Answer: (D) m-Nitrophenol
Year: 2004 | Set: 3
Q45. Which of the following shows maximum reactivity towards Lucas reagent?
(A) 1° Alcohol
(B) 2° Alcohol
(C) 3° Alcohol
(D) Allyl alcohol
Answer: (C) 3° Alcohol
Year: 2003 | Set: S
Q46. The product of the reaction:
Phenol + CH₃COCl → (in presence of NaOH)
(A) o-Hydroxyacetophenone
(B) p-Hydroxyacetophenone
(C) Both A and B
(D) Benzyl alcohol
Answer: (C) Both A and B
Year: 2003 | Set: 2
Q47. Which of the following on oxidation gives an acid and not a ketone?
(A) Ethanol
(B) 2-Propanol
(C) 2-Butanol
(D) Isopropyl alcohol
Answer: (A) Ethanol
Year: 2002 | Set: Q
Q48. Which one will undergo electrophilic substitution most easily?
(A) Benzene
(B) Phenol
(C) Toluene
(D) Nitrobenzene
Answer: (B) Phenol
Year: 2002 | Set: 1
Q49. Ethanol reacts with Na to form:
(A) CH₃CH₂ONa + H₂
(B) CH₃CH₂OH₂⁺
(C) CH₃CH₂OCH₂CH₃
(D) CH₄ + NaOH
Answer: (A) CH₃CH₂ONa + H₂
Year: 2001 | Set: M
Q50. Phenol reacts with bromine water to give:
(A) Bromobenzene
(B) o-Bromophenol
(C) 2,4,6-Tribromophenol
(D) 3-Bromophenol
Answer: (C) 2,4,6-Tribromophenol
Year: 2001 | Set: 2
Q51. Which of the following ethers will show peroxide formation on standing in air?
(A) Diethyl ether
(B) Methanol
(C) Ethanol
(D) Phenol
Answer: (A) Diethyl ether
Year: 2000 | Set: L
Q52. Which of the following is used in the manufacture of bakelite resin?
(A) Phenol
(B) Benzyl alcohol
(C) Formaldehyde
(D) Both A and C
Answer: (D) Both A and C
Year: 2000 | Set: 3
Q53. Which of the following is more soluble in water?
(A) Phenol
(B) Toluene
(C) Benzene
(D) Aniline
Answer: (A) Phenol
Year: 1999 | Set: N
Q54. Which compound shows intermolecular hydrogen bonding?
(A) Diethyl ether
(B) Acetone
(C) Phenol
(D) Benzene
Answer: (C) Phenol
Year: 1999 | Set: 1
Q55. Which of the following can be prepared by hydrolysis of Grignard reagent?
(A) Primary alcohol
(B) Tertiary alcohol
(C) Secondary alcohol
(D) All of these
Answer: (D) All of these
Year: 1998 | Set: S
Q56. Which reaction will lead to formation of anisole?
(A) C₆H₅ONa + CH₃I
(B) C₆H₅OH + CH₃Cl
(C) C₆H₅OH + CH₃OH
(D) C₆H₅Br + NaOCH₃
Answer: (A) C₆H₅ONa + CH₃I
Year: 1998 | Set: 2
Q57. Phenol undergoes electrophilic substitution due to:
(A) Electron withdrawing −OH group
(B) Lone pair donation by oxygen
(C) Delocalisation of lone pair
(D) Both B and C
Answer: (D) Both B and C
Year: 1997 | Set: Q
Q58. The major product in the reaction of ethanol with conc. H₂SO₄ at 413 K is:
(A) Ethene
(B) Acetaldehyde
(C) Diethyl ether
(D) Ethanoic acid
Answer: (C) Diethyl ether
Year: 1997 | Set: 3
Q59. What is the hybridisation of oxygen atom in alcohol?
(A) sp
(B) sp²
(C) sp³
(D) dsp²
Answer: (C) sp³
Year: 1996 | Set: M
Q60. Which compound gives effervescence with NaHCO₃?
(A) Ethanol
(B) Phenol
(C) Anisole
(D) Acetic acid
Answer: (D) Acetic acid
Year: 1996 | Set: 1
Q61. Phenol reacts with conc. HNO₃ to form:
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) 2,4,6-Trinitrophenol
Answer: (D) 2,4,6-Trinitrophenol
Year: 1995 | Set: S
Q62. Ether molecules are:
(A) Polar
(B) Non-polar
(C) Ionic
(D) Aromatic
Answer: (A) Polar
Year: 1995 | Set: 2
Q63. Which of the following has highest acidity?
(A) Ethanol
(B) Phenol
(C) Acetic acid
(D) Aniline
Answer: (C) Acetic acid
Year: 1994 | Set: Q
Q64. Oxidation of primary alcohol gives:
(A) Ketone
(B) Carboxylic acid
(C) Ether
(D) Alkene
Answer: (B) Carboxylic acid
Year: 1994 | Set: 1
Q65. Which of the following gives violet colour with FeCl₃?
(A) Acetic acid
(B) Benzaldehyde
(C) Phenol
(D) Acetophenone
Answer: (C) Phenol
Year: 1993 | Set: M
Q66. Dehydration of alcohol using conc. H₂SO₄ at 413 K gives:
(A) Ether
(B) Alkene
(C) Aldehyde
(D) Acid
Answer: (A) Ether
Year: 1993 | Set: 2
Q67. The compound with highest boiling point is:
(A) Diethyl ether
(B) Ethanol
(C) Propane
(D) Acetone
Answer: (B) Ethanol
Year: 1992 | Set: L
Q68. Oxidation of ethanol with hot alkaline KMnO₄ gives:
(A) Acetaldehyde
(B) Acetic acid
(C) Methane
(D) Methanol
Answer: (B) Acetic acid
Year: 1992 | Set: 3
Q69. Ethanol gives effervescence with:
(A) NaHCO₃
(B) NaOH
(C) Na metal
(D) H₂SO₄
Answer: (C) Na metal
Year: 1991 | Set: Q
Q70. In Reimer–Tiemann reaction, the intermediate is:
(A) Carbene
(B) Carbanion
(C) Carbonium ion
(D) Carboxylate
Answer: (A) Carbene
Year: 1991 | Set: 2
Q71. Phenol on oxidation with chromic acid gives:
(A) Cyclohexanone
(B) p-Benzoquinone
(C) Catechol
(D) Hydroquinone
Answer: (B) p-Benzoquinone
Year: 1990 | Set: M
Q72. Which of the following has the highest tendency to form intramolecular hydrogen bond?
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) Phenol
Answer: (A) o-Nitrophenol
Year: 1990 | Set: 1
Q73. Which compound liberates hydrogen when treated with Na metal?
(A) Diethyl ether
(B) Acetophenone
(C) Phenol
(D) Benzene
Answer: (C) Phenol
Year: 1990 | Set: S
Q74. Ethanol when passed over heated Al₂O₃ at 443 K gives:
(A) Acetylene
(B) Ethene
(C) Acetone
(D) Diethyl ether
Answer: (B) Ethene
Year: 1990 | Set: 3
Q75. Phenol does not undergo:
(A) Kolbe’s reaction
(B) Friedel–Crafts reaction
(C) Nitration
(D) Aldol condensation
Answer: (D) Aldol condensation
Year: 1990 | Set: N
Q76. Which of the following compounds does not show acidic character?
(A) Ethanol
(B) Phenol
(C) Acetic acid
(D) Aniline
Answer: (D) Aniline
Year: 1989 | Set: Z
Q77. The compound which reacts with neutral FeCl₃ to give violet colour is:
(A) Aniline
(B) Phenol
(C) Benzyl alcohol
(D) Acetaldehyde
Answer: (B) Phenol
Year: 1989 | Set: 1
Q78. The reagent used to convert phenol to anisole is:
(A) CH₃I/NaOH
(B) CH₃Cl/AlCl₃
(C) CH₃COCl/NaOH
(D) CH₃MgBr
Answer: (A) CH₃I/NaOH
Year: 1988 | Set: Q
Q79. Ethanol when reacted with hot acidified K₂Cr₂O₇ gives:
(A) Acetone
(B) Acetaldehyde
(C) Acetic acid
(D) Formic acid
Answer: (C) Acetic acid
Year: 1988 | Set: 2
Q80. The compound which does not respond to Lucas test is:
(A) Tert-butyl alcohol
(B) Sec-butyl alcohol
(C) Ethanol
(D) Isopropyl alcohol
Answer: (C) Ethanol
Year: 1988 | Set: M
Q81. In Kolbe’s reaction, phenol reacts with:
(A) CO₂ and acid
(B) CO₂ and base
(C) CO₂ and H₂
(D) CO and NaOH
Answer: (B) CO₂ and base
Year: 1987 | Set: L
Q82. Which of the following shows maximum boiling point?
(A) Diethyl ether
(B) Ethanol
(C) Acetone
(D) Ethene
Answer: (B) Ethanol
Year: 1987 | Set: 3
Q83. Which of the following undergoes electrophilic substitution reaction most readily?
(A) Benzene
(B) Phenol
(C) Nitrobenzene
(D) Toluene
Answer: (B) Phenol
Year: 1987 | Set: N
Q84. Alcohols are generally more soluble in water than hydrocarbons because of:
(A) Ionic bonding
(B) Hydrogen bonding
(C) Vander Waals forces
(D) Dipole-dipole interactions
Answer: (B) Hydrogen bonding
Year: 1986 | Set: 1
Q85. The Williamson synthesis does not work well when the alkyl halide is:
(A) Primary
(B) Secondary
(C) Tertiary
(D) Methyl
Answer: (C) Tertiary
Year: 1986 | Set: S
Q86. Phenol + conc. HNO₃ gives:
(A) m-Nitrophenol
(B) 2,4,6-Trinitrophenol
(C) Nitrobenzene
(D) o-Nitrophenol
Answer: (B) 2,4,6-Trinitrophenol
Year: 1986 | Set: 3
Q87. Which of the following cannot be prepared by oxidation of primary alcohol?
(A) Aldehyde
(B) Carboxylic acid
(C) Ketone
(D) None of these
Answer: (C) Ketone
Year: 1985 | Set: Q
Q88. On heating phenol with Zn dust, the product formed is:
(A) Benzene
(B) Benzoic acid
(C) Phenyl zinc
(D) Phenyl acetate
Answer: (A) Benzene
Year: 1985 | Set: 2
Q89. Reimer–Tiemann reaction is used to convert phenol into:
(A) Anisole
(B) Salicylaldehyde
(C) Salicylic acid
(D) Picric acid
Answer: (B) Salicylaldehyde
Year: 1985 | Set: M
Q90. The oxidation of secondary alcohol gives:
(A) Primary alcohol
(B) Ketone
(C) Aldehyde
(D) Acid
Answer: (B) Ketone
Year: 1985 | Set: 1
Q91. Which alcohol gives turbidity immediately with Lucas reagent?
(A) Ethanol
(B) 1-Propanol
(C) 2-Propanol
(D) 2-Methyl-2-propanol
Answer: (D) 2-Methyl-2-propanol
Year: 1984 | Set: N
Q92. The major product when phenol reacts with CHCl₃ and NaOH is:
(A) Benzaldehyde
(B) Anisole
(C) Salicylaldehyde
(D) Salicylic acid
Answer: (C) Salicylaldehyde
Year: 1984 | Set: 3
Q93. Phenol on treatment with Br₂ in CS₂ gives:
(A) o-Bromophenol
(B) p-Bromophenol
(C) o- and p-Bromophenol
(D) 2,4,6-Tribromophenol
Answer: (C) o- and p-Bromophenol
Year: 1983 | Set: M
Q94. The major product of dehydration of ethanol at 443 K is:
(A) Ethene
(B) Diethyl ether
(C) Acetaldehyde
(D) Acetic acid
Answer: (A) Ethene
Year: 1983 | Set: 2
Q95. Which of the following will be most reactive towards electrophilic substitution?
(A) Benzene
(B) Nitrobenzene
(C) Toluene
(D) Phenol
Answer: (D) Phenol
Year: 1982 | Set: S
Q96. Which alcohol is used as an antiseptic in hand sanitizers?
(A) Methanol
(B) Ethanol
(C) Phenol
(D) Benzyl alcohol
Answer: (B) Ethanol
Year: 2021 | Set: M
Q97. Boiling point order is:
(A) Ethanol > Methanol > Phenol
(B) Phenol > Ethanol > Ether
(C) Ether > Alcohol > Phenol
(D) Alcohol > Ether > Alkane
Answer: (B) Phenol > Ethanol > Ether
Year: 2018 | Set: 2
Q98. On oxidation of ethanol, the product formed is:
(A) Methanol
(B) Acetone
(C) Ethanoic acid
(D) Ethanal
Answer: (C) Ethanoic acid
Year: 2015 | Set: R
Q99. Phenol + NaOH followed by CO₂ and acidification gives:
(A) Anisole
(B) Salicylaldehyde
(C) Salicylic acid
(D) o-Hydroxyacetophenone
Answer: (C) Salicylic acid
Year: 2019 | Set: Q
Q100. Which of the following does not give violet colour with neutral FeCl₃?
(A) o-Cresol
(B) Phenol
(C) Salicylic acid
(D) Ethanol
Answer: (D) Ethanol
Year: 2025 | Set: Z
————————————————————————————————————————————————————————————————————————————
JEE MAINS QUESTIONS FROM THIS LESSON
Q1. Which of the following will react fastest with Lucas reagent?
(A) 1-Butanol
(B) 2-Butanol
(C) 2-Methyl-2-propanol
(D) Ethanol
Answer: (C) 2-Methyl-2-propanol
Year: 2024 | Shift: 2 | Set: A
Q2. Phenol reacts with bromine water to give:
(A) Bromobenzene
(B) 2-Bromophenol
(C) 2,4,6-Tribromophenol
(D) No reaction
Answer: (C) 2,4,6-Tribromophenol
Year: 2024 | Shift: 1 | Set: C
Q3. Kolbe’s reaction involves the formation of:
(A) Salicylaldehyde
(B) Salicylic acid
(C) Picric acid
(D) Anisole
Answer: (B) Salicylic acid
Year: 2023 | Shift: 2 | Set: B
Q4. The acidic strength of phenol is due to:
(A) Electron releasing −OH group
(B) Resonance stabilization of phenoxide ion
(C) Hydrogen bonding
(D) Inductive effect of −OH
Answer: (B) Resonance stabilization of phenoxide ion
Year: 2023 | Shift: 1 | Set: A
Q5. Reimer–Tiemann reaction involves the use of:
(A) CHCl₃ + NaOH
(B) CH₃COCl
(C) Br₂ + H₂O
(D) NH₃ + CHCl₃
Answer: (A) CHCl₃ + NaOH
Year: 2022 | Shift: 2 | Set: D
Q6. Which of the following ethers undergo cleavage by HI most easily?
(A) Diethyl ether
(B) Methyl phenyl ether
(C) Ethyl phenyl ether
(D) Methyl tert-butyl ether
Answer: (D) Methyl tert-butyl ether
Year: 2022 | Shift: 1 | Set: A
Q7. Which compound gives effervescence with NaHCO₃?
(A) Phenol
(B) Ethanol
(C) Acetic acid
(D) Anisole
Answer: (C) Acetic acid
Year: 2021 | Shift: 1 | Set: C
Q8. The electrophile in Kolbe’s reaction is:
(A) CHCl₂⁺
(B) :CCl₂
(C) Cl⁺
(D) CO₂
Answer: (D) CO₂
Year: 2021 | Shift: 2 | Set: B
Q9. Which of the following is used for the preparation of anisole?
(A) C₆H₅OH + CH₃Cl
(B) C₆H₅ONa + CH₃I
(C) C₆H₅Cl + CH₃ONa
(D) C₆H₅OH + CH₃OH
Answer: (B) C₆H₅ONa + CH₃I
Year: 2020 | Shift: 2 | Set: D
Q10. Lucas test distinguishes alcohols based on:
(A) Rate of reduction
(B) Rate of turbidity formation
(C) Colour change
(D) Rate of oxidation
Answer: (B) Rate of turbidity formation
Year: 2020 | Shift: 1 | Set: A
Q11. What is formed when phenol is treated with CHCl₃ and NaOH?
(A) Benzoic acid
(B) Anisole
(C) o-Hydroxybenzaldehyde
(D) Benzaldehyde
Answer: (C) o-Hydroxybenzaldehyde
Year: 2019 | Shift: 1 | Set: B
Q12. Which of the following does not give violet colour with FeCl₃?
(A) Phenol
(B) o-Cresol
(C) Salicylic acid
(D) Benzyl alcohol
Answer: (D) Benzyl alcohol
Year: 2019 | Shift: 2 | Set: C
Q13. Which of the following alcohols undergoes dehydration most easily?
(A) 1-Butanol
(B) 2-Butanol
(C) 2-Methyl-2-propanol
(D) Ethanol
Answer: (C) 2-Methyl-2-propanol
Year: 2018 | Shift: 1 | Set: A
Q14. The compound that gives white ppt with Br₂ water is:
(A) Anisole
(B) Benzene
(C) Phenol
(D) Benzyl alcohol
Answer: (C) Phenol
Year: 2018 | Shift: 2 | Set: D
Q15. An ether is more volatile than its isomeric alcohol due to:
(A) Strong hydrogen bonding
(B) Lack of hydrogen bonding
(C) Dipole-dipole interaction
(D) Vander Waals forces
Answer: (B) Lack of hydrogen bonding
Year: 2017 | Shift: 1 | Set: C
Q16. Which of the following is most acidic?
(A) Ethanol
(B) Phenol
(C) o-Nitrophenol
(D) p-Methoxyphenol
Answer: (C) o-Nitrophenol
Year: 2017 | Shift: 2 | Set: A
Q17. Williamson synthesis is not suitable for preparing:
(A) Anisole
(B) tert-Butyl methyl ether
(C) Diethyl ether
(D) Ethyl phenyl ether
Answer: (B) tert-Butyl methyl ether
Year: 2016 | Shift: 2 | Set: D
Q18. Boiling point order is:
(A) Ether > Alcohol > Phenol
(B) Phenol > Alcohol > Ether
(C) Alcohol > Ether > Alkane
(D) Alcohol > Phenol > Ether
Answer: (B) Phenol > Alcohol > Ether
Year: 2016 | Shift: 1 | Set: A
Q19. Which of the following does not undergo SN2 reaction in Williamson synthesis?
(A) Benzyl bromide
(B) Methyl iodide
(C) 1-Bromopropane
(D) tert-Butyl bromide
Answer: (D) tert-Butyl bromide
Year: 2015 | Shift: 2 | Set: C
Q20. The hybridisation of oxygen atom in alcohol is:
(A) sp
(B) sp²
(C) sp³
(D) dsp²
Answer: (C) sp³
Year: 2015 | Shift: 1 | Set: B
Q21. Which of the following does not evolve H₂ gas with sodium?
(A) Phenol
(B) Ethanol
(C) Methanol
(D) Diethyl ether
Answer: (D) Diethyl ether
Year: 2014 | Shift: 2 | Set: D
Q22. When ethanol reacts with acidified K₂Cr₂O₇, the product is:
(A) Acetone
(B) Acetic acid
(C) Methane
(D) Methanol
Answer: (B) Acetic acid
Year: 2014 | Shift: 1 | Set: A
Q23. Phenol on treatment with Zn gives:
(A) Benzene
(B) Aniline
(C) Benzaldehyde
(D) Toluene
Answer: (A) Benzene
Year: 2013 | Shift: 2 | Set: C
Q24. Which compound gives violet colour with neutral FeCl₃?
(A) Acetophenone
(B) Phenol
(C) Acetic acid
(D) Ethanol
Answer: (B) Phenol
Year: 2013 | Shift: 1 | Set: B
Q25. The reagent used for the oxidation of secondary alcohol to ketone is:
(A) PCC
(B) LiAlH₄
(C) NaBH₄
(D) Zn/acid
Answer: (A) PCC
Year: 2012 | Shift: 2 | Set: A
Q26. Which of the following reacts fastest with HBr?
(A) Methanol
(B) Ethanol
(C) Isopropanol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Year: 2012 | Shift: 1 | Set: C
Q27. The oxidation of 1° alcohol gives:
(A) Aldehyde
(B) Ketone
(C) Ether
(D) Alkene
Answer: (A) Aldehyde
Year: 2011 | Shift: 2 | Set: A
Q28. In Reimer–Tiemann reaction, the intermediate is:
(A) CHCl₃
(B) :CCl₂
(C) Cl₂
(D) CHCl₂⁺
Answer: (B) :CCl₂
Year: 2011 | Shift: 1 | Set: B
Q29. The acidity of phenol is due to:
(A) Resonance stabilization
(B) Hydrogen bonding
(C) High electronegativity of O
(D) Polarisation of O–H bond
Answer: (A) Resonance stabilization
Year: 2010 | Shift: 2 | Set: D
Q30. Which of the following compounds gives positive Lucas test immediately?
(A) 1° Alcohol
(B) 2° Alcohol
(C) 3° Alcohol
(D) Phenol
Answer: (C) 3° Alcohol
Year: 2010 | Shift: 1 | Set: A
Q31. Which of the following will form an alkene most easily on dehydration?
(A) Ethanol
(B) 2-Butanol
(C) 2-Methyl-2-butanol
(D) Methanol
Answer: (C) 2-Methyl-2-butanol
Year: 2009 | Shift: 2 | Set: B
Q32. When phenol is treated with conc. HNO₃, the major product is:
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) 2,4,6-Trinitrophenol
Answer: (D) 2,4,6-Trinitrophenol
Year: 2009 | Shift: 1 | Set: C
Q33. Which among the following cannot be oxidised to carboxylic acid?
(A) Ethanol
(B) Methanol
(C) Propan-1-ol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Year: 2008 | Shift: 2 | Set: A
Q34. Williamson synthesis is suitable for:
(A) Anisole
(B) Diphenyl ether
(C) tert-Butyl methyl ether
(D) Ethanol
Answer: (A) Anisole
Year: 2008 | Shift: 1 | Set: D
Q35. Which of the following has lowest boiling point?
(A) Ethanol
(B) Diethyl ether
(C) Water
(D) Methanol
Answer: (B) Diethyl ether
Year: 2007 | Shift: 2 | Set: C
Q36. An organic compound C₇H₈O gives violet colour with FeCl₃ and a white precipitate with bromine water. The compound is:
(A) Toluene
(B) Benzyl alcohol
(C) o-Cresol
(D) Benzaldehyde
Answer: (C) o-Cresol
Year: 2007 | Shift: 1 | Set: A
Q37. Phenol on treatment with NaOH gives:
(A) Sodium phenoxide
(B) Sodium benzoate
(C) Anisole
(D) Salicylaldehyde
Answer: (A) Sodium phenoxide
Year: 2006 | Shift: 2 | Set: D
Q38. Which of the following does not give hydrogen with sodium?
(A) Ethanol
(B) Phenol
(C) Diethyl ether
(D) Water
Answer: (C) Diethyl ether
Year: 2006 | Shift: 1 | Set: B
Q39. Dehydration of ethanol gives:
(A) Acetaldehyde
(B) Ethene
(C) Acetic acid
(D) Ethanol
Answer: (B) Ethene
Year: 2005 | Shift: 2 | Set: C
Q40. Which will not undergo SN2 in Williamson synthesis?
(A) Benzyl bromide
(B) 1-Bromopropane
(C) tert-Butyl bromide
(D) Methyl iodide
Answer: (C) tert-Butyl bromide
Year: 2005 | Shift: 1 | Set: A
Q41. Which of the following does not give colour with neutral FeCl₃?
(A) Phenol
(B) Salicylic acid
(C) Anisole
(D) o-Cresol
Answer: (C) Anisole
Year: 2004 | Shift: 2 | Set: B
Q42. Phenol reacts with Zn to form:
(A) Benzene
(B) Benzaldehyde
(C) Aniline
(D) Toluene
Answer: (A) Benzene
Year: 2004 | Shift: 1 | Set: D
Q43. Which alcohol cannot be oxidised to carboxylic acid?
(A) Ethanol
(B) Methanol
(C) Isopropanol
(D) Propanol
Answer: (C) Isopropanol
Year: 2003 | Shift: 2 | Set: A
Q44. Williamson synthesis of methyl tert-butyl ether is best done using:
(A) Methyl bromide + sodium tert-butoxide
(B) tert-Butyl bromide + sodium methoxide
(C) tert-Butyl alcohol + CH₃I
(D) CH₃ONa + (CH₃)₃CCl
Answer: (A) Methyl bromide + sodium tert-butoxide
Year: 2003 | Shift: 1 | Set: C
Q45. Reaction of phenol with CO₂ and NaOH gives:
(A) Salicylaldehyde
(B) Salicylic acid
(C) Acetophenone
(D) Anisole
Answer: (B) Salicylic acid
Year: 2002 | Shift: 2 | Set: B
Q46. Phenol is more acidic than ethanol because:
(A) Phenoxide ion is resonance stabilised
(B) Phenol has lower pKa
(C) Phenol is aromatic
(D) Ethanol forms stronger H-bonds
Answer: (A) Phenoxide ion is resonance stabilised
Year: 2002 | Shift: 1 | Set: A
Q47. Which of the following gives an SN1 product in alcohol + HX reaction?
(A) Methanol
(B) Ethanol
(C) 2-Butanol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Year: 2001 | Shift: 2 | Set: C
Q48. The alcohol that shows positive Lucas test immediately is:
(A) Ethanol
(B) Isopropanol
(C) tert-Butyl alcohol
(D) 1-Propanol
Answer: (C) tert-Butyl alcohol
Year: 2001 | Shift: 1 | Set: D
Q49. The dehydration of ethanol gives:
(A) Diethyl ether at 413 K
(B) Ethene at 443 K
(C) Acetic acid at 353 K
(D) Aldehyde at 400 K
Answer: (B) Ethene at 443 K
Year: 2001 | Shift: 2 | Set: B
Q50. Which compound liberates H₂ gas with Na?
(A) Anisole
(B) Phenol
(C) Acetone
(D) Benzene
Answer: (B) Phenol
Year: 2001 | Shift: 1 | Set: A
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
🔷 JEE Advanced Paper 1 (Q1–Q17)
Q1. Which of the following alcohols reacts fastest with Lucas reagent?
(A) 1-Butanol
(B) 2-Butanol
(C) 2-Methyl-2-propanol
(D) Benzyl alcohol
Answer: (C) 2-Methyl-2-propanol
Year: 2025 | Paper: 1 | Set: 1
Q2. Which among the following does NOT give a violet colour with neutral FeCl₃?
(A) Phenol
(B) o-Cresol
(C) Salicylic acid
(D) Anisole
Answer: (D) Anisole
Year: 2024 | Paper: 1 | Set: 2
Q3. The electrophile in the Kolbe reaction is:
(A) :CCl₂
(B) CO₂
(C) CHCl₃
(D) Cl⁺
Answer: (B) CO₂
Year: 2023 | Paper: 1 | Set: 1
Q4. Phenol on treatment with Zn gives:
(A) Benzene
(B) Benzaldehyde
(C) Aniline
(D) Toluene
Answer: (A) Benzene
Year: 2023 | Paper: 1 | Set: 2
Q5. Which alcohol undergoes dehydration most easily?
(A) 1-Butanol
(B) 2-Butanol
(C) 2-Methyl-2-propanol
(D) Ethanol
Answer: (C) 2-Methyl-2-propanol
Year: 2022 | Paper: 1 | Set: 2
Q6. Reimer–Tiemann reaction involves formation of:
(A) Salicylic acid
(B) o-Hydroxybenzaldehyde
(C) Benzoic acid
(D) Anisole
Answer: (B) o-Hydroxybenzaldehyde
Year: 2022 | Paper: 1 | Set: 1
Q7. Which is most acidic?
(A) Ethanol
(B) Phenol
(C) o-Nitrophenol
(D) p-Cresol
Answer: (C) o-Nitrophenol
Year: 2021 | Paper: 1 | Set: 1
Q8. Williamson synthesis is unsuitable for which of the following combinations?
(A) CH₃ONa + C₂H₅Br
(B) C₆H₅ONa + CH₃Br
(C) C(CH₃)₃Br + CH₃ONa
(D) C₂H₅ONa + CH₃Br
Answer: (C) C(CH₃)₃Br + CH₃ONa
Year: 2021 | Paper: 1 | Set: 2
Q9. Phenol gives white precipitate with:
(A) Br₂ in CS₂
(B) Br₂ water
(C) Dil. HNO₃
(D) Acetic anhydride
Answer: (B) Br₂ water
Year: 2020 | Paper: 1 | Set: 1
Q10. The mechanism involved in dehydration of alcohol to alkene is:
(A) E2
(B) SN1
(C) SN2
(D) E1
Answer: (D) E1
Year: 2020 | Paper: 1 | Set: 2
Q11. Ethanol is oxidized by acidified K₂Cr₂O₇ to:
(A) Acetaldehyde
(B) Ethene
(C) Acetic acid
(D) Methanol
Answer: (C) Acetic acid
Year: 2019 | Paper: 1 | Set: 2
Q12. Phenol reacts with aqueous NaOH to form:
(A) Sodium phenoxide
(B) Benzene
(C) Phenyl acetate
(D) Salicylaldehyde
Answer: (A) Sodium phenoxide
Year: 2019 | Paper: 1 | Set: 1
Q13. Ether is more volatile than alcohol due to:
(A) Dipole-dipole interaction
(B) Lack of H-bonding
(C) Lower molecular mass
(D) High symmetry
Answer: (B) Lack of H-bonding
Year: 2018 | Paper: 1 | Set: 2
Q14. Which of the following alcohols gives a ketone on oxidation?
(A) Ethanol
(B) Methanol
(C) 2-Propanol
(D) Benzyl alcohol
Answer: (C) 2-Propanol
Year: 2018 | Paper: 1 | Set: 1
Q15. Which compound gives effervescence with NaHCO₃ but not with NaOH?
(A) Acetic acid
(B) Phenol
(C) Alcohol
(D) Benzaldehyde
Answer: (A) Acetic acid
Year: 2017 | Paper: 1 | Set: 1
Q16. Lucas reagent gives fastest turbidity with:
(A) Ethanol
(B) 1-Propanol
(C) 2-Propanol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Year: 2017 | Paper: 1 | Set: 2
Q17. Which of the following forms violet colour with FeCl₃?
(A) Acetophenone
(B) Phenol
(C) Benzyl alcohol
(D) Ethanol
Answer: (B) Phenol
Year: 2016 | Paper: 1 | Set: 1
🔷 JEE Advanced Paper 2 (Q18–Q34)
Q18. On nitration with dilute HNO₃, phenol gives:
(A) o-Nitrophenol
(B) m-Nitrophenol
(C) 2,4-Dinitrophenol
(D) Nitrobenzene
Answer: (A) o-Nitrophenol
Year: 2025 | Paper: 2 | Set: 2
Q19. Boiling point order is:
(A) Water > Phenol > Diethyl ether
(B) Phenol > Diethyl ether > Water
(C) Diethyl ether > Phenol > Water
(D) Phenol > Water > Diethyl ether
Answer: (A) Water > Phenol > Diethyl ether
Year: 2024 | Paper: 2 | Set: 1
Q20. Dehydration of ethanol gives:
(A) Ethene
(B) Acetic acid
(C) Acetaldehyde
(D) Diethyl ether
Answer: (A) Ethene
Year: 2024 | Paper: 2 | Set: 2
Q21. Which is most reactive toward electrophilic substitution?
(A) Benzene
(B) Toluene
(C) Phenol
(D) Anisole
Answer: (C) Phenol
Year: 2023 | Paper: 2 | Set: 2
Q22. Which among the following is least acidic?
(A) Phenol
(B) Ethanol
(C) o-Nitrophenol
(D) p-Nitrophenol
Answer: (B) Ethanol
Year: 2023 | Paper: 2 | Set: 1
Q23. Which of the following will not give substitution with HI?
(A) Diethyl ether
(B) Ethyl methyl ether
(C) Anisole
(D) tert-Butyl methyl ether
Answer: (C) Anisole
Year: 2022 | Paper: 2 | Set: 1
Q24. In Williamson synthesis, suitable combination for anisole is:
(A) CH₃ONa + C₆H₅Br
(B) CH₃Br + C₆H₅ONa
(C) CH₃Cl + C₆H₅OH
(D) C₆H₅Cl + CH₃ONa
Answer: (B) CH₃Br + C₆H₅ONa
Year: 2022 | Paper: 2 | Set: 2
Q25. Which alcohol is most resistant to oxidation?
(A) Ethanol
(B) 2-Propanol
(C) tert-Butyl alcohol
(D) 1-Butanol
Answer: (C) tert-Butyl alcohol
Year: 2021 | Paper: 2 | Set: 1
Q26. Which product is formed by Kolbe’s reaction?
(A) Benzoic acid
(B) o-Hydroxybenzaldehyde
(C) Salicylic acid
(D) Anisole
Answer: (C) Salicylic acid
Year: 2021 | Paper: 2 | Set: 2
Q27. Phenol reacts with CHCl₃ and NaOH to give:
(A) Anisole
(B) o-Hydroxybenzaldehyde
(C) Benzaldehyde
(D) Salicylic acid
Answer: (B) o-Hydroxybenzaldehyde
Year: 2020 | Paper: 2 | Set: 1
Q28. Lucas reagent is a mixture of:
(A) ZnCl₂ + conc. HCl
(B) FeCl₃ + HCl
(C) NaOH + HCl
(D) Zn + dilute HCl
Answer: (A) ZnCl₂ + conc. HCl
Year: 2020 | Paper: 2 | Set: 2
Q29. Which of the following gives substitution with HI?
(A) Anisole
(B) Phenol
(C) Ethyl methyl ether
(D) Toluene
Answer: (C) Ethyl methyl ether
Year: 2019 | Paper: 2 | Set: 1
Q30. Which of the following gives acidic hydrogen?
(A) Benzene
(B) Toluene
(C) Phenol
(D) Anisole
Answer: (C) Phenol
Year: 2019 | Paper: 2 | Set: 2
Q31. Which of the following is used as an anaesthetic?
(A) Diethyl ether
(B) Methanol
(C) Phenol
(D) Acetaldehyde
Answer: (A) Diethyl ether
Year: 2018 | Paper: 2 | Set: 1
Q32. Phenol reacts with acetic anhydride to give:
(A) Ester
(B) Ether
(C) Aldehyde
(D) Alcohol
Answer: (A) Ester
Year: 2018 | Paper: 2 | Set: 2
Q33. Which of the following is not formed in nitration of phenol using dilute HNO₃?
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) Water
Answer: (C) m-Nitrophenol
Year: 2017 | Paper: 2 | Set: 2
Q34. Which of the following has the lowest boiling point?
(A) Water
(B) Phenol
(C) Diethyl ether
(D) Ethanol
Answer: (C) Diethyl ether
Year: 2017 | Paper: 2 | Set: 1
————————————————————————————————————————————————————————————————————————————
PRACTICE SETS FROM THIS LESSON
Q1. The hybridisation of oxygen in alcohols is:
(A) sp
(B) sp²
(C) sp³
(D) sp³d
Answer: (C) sp³
Q2. Phenol reacts with bromine water to give:
(A) o-Bromophenol
(B) m-Bromophenol
(C) 2,4,6-Tribromophenol
(D) No reaction
Answer: (C) 2,4,6-Tribromophenol
Q3. Which alcohol gives immediate turbidity with Lucas reagent?
(A) Ethanol
(B) 1-Propanol
(C) 2-Propanol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Q4. The product obtained when phenol reacts with CHCl₃ and NaOH is:
(A) Salicylaldehyde
(B) Salicylic acid
(C) Anisole
(D) Benzaldehyde
Answer: (A) Salicylaldehyde
Q5. Williamson synthesis is unsuitable for:
(A) CH₃Br + C₂H₅ONa
(B) CH₃Br + C₆H₅ONa
(C) C(CH₃)₃Br + CH₃ONa
(D) CH₃CH₂Br + CH₃ONa
Answer: (C) C(CH₃)₃Br + CH₃ONa
Q6. Phenol is more acidic than ethanol due to:
(A) Higher molecular mass
(B) Inductive effect
(C) Resonance stabilization of phenoxide ion
(D) Electrophilic nature
Answer: (C) Resonance stabilization of phenoxide ion
Q7. Which of the following gives violet colour with FeCl₃?
(A) Ethanol
(B) Acetone
(C) Phenol
(D) Benzene
Answer: (C) Phenol
Q8. Which compound liberates H₂ gas on reaction with sodium?
(A) Acetone
(B) Diethyl ether
(C) Ethanol
(D) Anisole
Answer: (C) Ethanol
Q9. Kolbe’s reaction is used to prepare:
(A) Phenyl acetate
(B) Salicylic acid
(C) Anisole
(D) Benzyl alcohol
Answer: (B) Salicylic acid
Q10. Which of the following does not undergo Lucas test?
(A) Ethanol
(B) Isopropanol
(C) tert-Butyl alcohol
(D) Benzyl alcohol
Answer: (A) Ethanol
Q11. Which of the following has the highest boiling point?
(A) Diethyl ether
(B) Ethanol
(C) Phenol
(D) Propane
Answer: (C) Phenol
Q12. Alcohols react with carboxylic acids in presence of acid to form:
(A) Ethers
(B) Esters
(C) Aldehydes
(D) Ketones
Answer: (B) Esters
Q13. Dehydration of ethanol gives:
(A) Acetic acid
(B) Ethene
(C) Acetaldehyde
(D) Diethyl ether
Answer: (B) Ethene
Q14. An ether can be cleaved by:
(A) HCl
(B) HI
(C) Br₂
(D) H₂SO₄
Answer: (B) HI
Q15. The IUPAC name of CH₃CH₂OCH₂CH₃ is:
(A) Methoxyethane
(B) Ethyl methyl ether
(C) Ethoxyethane
(D) Diethyl ether
Answer: (C) Ethoxyethane
Q16. Which of the following does not form an ether in Williamson synthesis?
(A) C₆H₅ONa + CH₃Br
(B) CH₃ONa + CH₃CH₂Br
(C) CH₃CH₂ONa + C(CH₃)₃Br
(D) CH₃CH₂ONa + CH₃I
Answer: (C) CH₃CH₂ONa + C(CH₃)₃Br
Q17. Boiling point order is:
(A) Alcohol > Ether > Alkane
(B) Ether > Alcohol > Alkane
(C) Ether > Alkane > Alcohol
(D) Alkane > Alcohol > Ether
Answer: (A) Alcohol > Ether > Alkane
Q18. Which reaction introduces a −CHO group into phenol?
(A) Reimer–Tiemann reaction
(B) Kolbe’s reaction
(C) Friedel–Crafts reaction
(D) Esterification
Answer: (A) Reimer–Tiemann reaction
Q19. The functional group in phenol is:
(A) −COOH
(B) −OH
(C) −CHO
(D) −COOR
Answer: (B) −OH
Q20. Anisole is:
(A) Phenyl alcohol
(B) Methyl phenyl ether
(C) Benzaldehyde
(D) Ethyl phenol
Answer: (B) Methyl phenyl ether
Q21. The reaction between CH₃OH and HBr gives:
(A) CH₄
(B) CH₃Br
(C) CH₃OH₂⁺
(D) CH₃CH₂Br
Answer: (B) CH₃Br
Q22. Which of the following is aromatic?
(A) Ethanol
(B) Diethyl ether
(C) Phenol
(D) Acetone
Answer: (C) Phenol
Q23. Phenol is less soluble in water than ethanol due to:
(A) Larger hydrophobic part
(B) Lesser polarity
(C) No H-bonding
(D) Lower boiling point
Answer: (A) Larger hydrophobic part
Q24. Electrophilic substitution in phenol occurs mostly at:
(A) Meta position
(B) Ortho and para positions
(C) Only ortho position
(D) Only para position
Answer: (B) Ortho and para positions
Q25. Ether cleavage is most efficient with:
(A) HI
(B) HCl
(C) H₂O
(D) HNO₃
Answer: (A) HI
Q26. Williamson synthesis involves:
(A) SN1 reaction
(B) SN2 reaction
(C) E1 reaction
(D) E2 reaction
Answer: (B) SN2 reaction
Q27. The −OH group in phenol is:
(A) Electron withdrawing
(B) Electron donating
(C) Inert
(D) Neutral
Answer: (B) Electron donating
Q28. Which of the following gives white ppt with bromine water?
(A) Benzene
(B) Ethanol
(C) Anisole
(D) Phenol
Answer: (D) Phenol
Q29. Salicylic acid can be prepared from:
(A) Phenol + CO₂
(B) Phenol + CHCl₃
(C) Benzene + CO₂
(D) Phenol + NaHCO₃
Answer: (A) Phenol + CO₂
Q30. Which alcohol will undergo oxidation to ketone?
(A) Ethanol
(B) Methanol
(C) 2-Propanol
(D) Benzyl alcohol
Answer: (C) 2-Propanol
Q31. The major product of dehydration of ethanol at 413 K is:
(A) Ethene
(B) Diethyl ether
(C) Acetaldehyde
(D) Acetic acid
Answer: (B) Diethyl ether
Q32. Which compound will not undergo SN2 in Williamson synthesis?
(A) 1-Bromopropane
(B) Benzyl bromide
(C) tert-Butyl bromide
(D) Methyl iodide
Answer: (C) tert-Butyl bromide
Q33. Which of the following is not formed by oxidation of ethanol?
(A) Acetic acid
(B) Ethanal
(C) CO₂
(D) Methanol
Answer: (D) Methanol
Q34. Which of the following is more volatile?
(A) Ethanol
(B) Phenol
(C) Diethyl ether
(D) Water
Answer: (C) Diethyl ether
Q35. The product formed on heating phenol with phthalic anhydride in the presence of conc. H₂SO₄ is:
(A) Salicylaldehyde
(B) Anisole
(C) Bakelite
(D) Phenolphthalein
Answer: (D) Phenolphthalein
Q36. Which one of the following is most acidic?
(A) Ethanol
(B) Water
(C) Phenol
(D) Cyclohexanol
Answer: (C) Phenol
Q37. The order of reactivity of alcohols with Lucas reagent is:
(A) 1° > 2° > 3°
(B) 3° > 2° > 1°
(C) 2° > 3° > 1°
(D) 1° > 3° > 2°
Answer: (B) 3° > 2° > 1°
Q38. Phenol is less reactive towards nucleophilic substitution due to:
(A) Electron-donating −OH group
(B) Resonance stabilization
(C) Partial double bond character in C–O bond
(D) Hydrogen bonding
Answer: (C) Partial double bond character in C–O bond
Q39. How many isomeric ethers are possible with molecular formula C₄H₁₀O?
(A) 2
(B) 3
(C) 4
(D) 5
Answer: (B) 3
Q40. The reaction of phenol with zinc dust gives:
(A) Benzene
(B) Toluene
(C) Anisole
(D) Nitrobenzene
Answer: (A) Benzene
Q41. Which of the following undergoes intramolecular hydrogen bonding?
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) m-Nitrophenol
(D) o-Cresol
Answer: (A) o-Nitrophenol
Q42. Which of the following has maximum boiling point?
(A) Diethyl ether
(B) n-Butyl alcohol
(C) Isobutyl alcohol
(D) tert-Butyl alcohol
Answer: (B) n-Butyl alcohol
Q43. When anisole is heated with HI, the products are:
(A) Phenol and CH₃I
(B) Iodobenzene and CH₃OH
(C) Phenol and methyl alcohol
(D) Phenol and methyl iodide
Answer: (D) Phenol and methyl iodide
Q44. Diethyl ether reacts with excess HI to form:
(A) CH₃CH₂I
(B) CH₃CH₂OH
(C) CH₃CH₂OCH₂CH₃
(D) Ethane
Answer: (A) CH₃CH₂I
Q45. The acidity of phenol is due to:
(A) Formation of phenoxide ion
(B) Instability of phenol
(C) Low molecular weight
(D) Electronegativity of carbon
Answer: (A) Formation of phenoxide ion
Q46. The major product in the reaction of phenol with Br₂ in CS₂ (cold) is:
(A) o-Bromophenol
(B) m-Bromophenol
(C) p-Bromophenol
(D) 2,4,6-Tribromophenol
Answer: (C) p-Bromophenol
Q47. Ethanol can be distinguished from phenol by reaction with:
(A) FeCl₃
(B) Na
(C) NaOH
(D) Br₂ water
Answer: (A) FeCl₃
Q48. On heating phenol with CHCl₃ and aq. NaOH followed by acidification, the product formed is:
(A) Salicylic acid
(B) p-Cresol
(C) o-Hydroxybenzaldehyde
(D) Benzoic acid
Answer: (C) o-Hydroxybenzaldehyde
Q49. The compound that will not form hydrogen bond is:
(A) Ethanol
(B) Diethyl ether
(C) Phenol
(D) Water
Answer: (B) Diethyl ether
Q50. Which of the following is formed when CH₃OH is treated with Zn at 573 K?
(A) Methanal
(B) Methane
(C) Methanoic acid
(D) Methyl iodide
Answer: (B) Methane
Q51. Which will react fastest in dehydration to form alkene?
(A) Ethanol
(B) 2-Propanol
(C) 2-Methyl-2-propanol
(D) 1-Propanol
Answer: (C) 2-Methyl-2-propanol
Q52. The number of moles of KMnO₄ required to oxidize 1 mol of ethylene glycol completely is:
(A) 1
(B) 2
(C) 3
(D) 4
Answer: (B) 2
Q53. Boiling points of ethers are:
(A) Higher than alcohols
(B) Equal to alcohols
(C) Lower than alcohols
(D) Same as hydrocarbons
Answer: (C) Lower than alcohols
Q54. Which pair will give an ether on reaction?
(A) C₂H₅ONa + CH₃Br
(B) C₆H₅ONa + CH₃Br
(C) C(CH₃)₃Br + C₂H₅ONa
(D) C₆H₅Br + CH₃ONa
Answer: (A) C₂H₅ONa + CH₃Br
Q55. Phenol can be converted into salicylic acid by:
(A) Reimer–Tiemann reaction
(B) Kolbe’s reaction
(C) Friedel–Crafts reaction
(D) Acetylation
Answer: (B) Kolbe’s reaction
Q56. Which alcohol on oxidation does not give a carbonyl compound?
(A) Ethanol
(B) Methanol
(C) 2-Propanol
(D) 2-Methyl-2-propanol
Answer: (D) 2-Methyl-2-propanol
Q57. Which statement is true about phenol?
(A) Acts only as a base
(B) Can form esters with acid
(C) Cannot show resonance
(D) Does not react with acid chlorides
Answer: (B) Can form esters with acid
Q58. p-Nitrophenol is more acidic than phenol due to:
(A) Electron-donating NO₂
(B) Electron-withdrawing NO₂
(C) Hyperconjugation
(D) +I effect
Answer: (B) Electron-withdrawing NO₂
Q59. An ether reacts with HI to give phenol and alkyl iodide. Ether is:
(A) CH₃OC₂H₅
(B) C₆H₅OCH₃
(C) CH₃CH₂OCH₃
(D) C₆H₅CH₂OCH₃
Answer: (B) C₆H₅OCH₃
Q60. How many moles of ethanol are needed to produce one mole of diethyl ether?
(A) 1
(B) 2
(C) 3
(D) 4
Answer: (B) 2
Q61. Which of the following ethers has highest boiling point?
(A) Dimethyl ether
(B) Diethyl ether
(C) Ethyl propyl ether
(D) Methyl tert-butyl ether
Answer: (C) Ethyl propyl ether
Q62. The acid strength order is:
(A) p-Cresol > Phenol > p-Nitrophenol
(B) p-Nitrophenol > Phenol > p-Cresol
(C) Phenol > p-Nitrophenol > p-Cresol
(D) Phenol > p-Cresol > p-Nitrophenol
Answer: (B) p-Nitrophenol > Phenol > p-Cresol
Q63. Ethanol on reaction with Na gives:
(A) CH₄
(B) H₂
(C) C₂H₄
(D) NaOH
Answer: (B) H₂
Q64. Oxidation of benzyl alcohol gives:
(A) Benzene
(B) Benzaldehyde
(C) Benzoic acid
(D) Toluene
Answer: (B) Benzaldehyde
Q65. Refluxing ethanol with acetic acid in presence of conc. H₂SO₄ gives:
(A) Acetaldehyde
(B) Ethyl acetate
(C) Acetamide
(D) Propanol
Answer: (B) Ethyl acetate
Q66. Which of the following undergoes Friedel–Crafts reaction easily?
(A) Phenol
(B) Nitrobenzene
(C) Benzoic acid
(D) Benzaldehyde
Answer: (A) Phenol
Q67. Sodium ethoxide reacts with water to form:
(A) Sodium hydroxide
(B) Acetic acid
(C) Ethanol
(D) Acetaldehyde
Answer: (C) Ethanol
Q68. Phenol on treatment with dil. HNO₃ at room temperature gives:
(A) o-Nitrophenol
(B) p-Nitrophenol
(C) Both (A) and (B)
(D) m-Nitrophenol
Answer: (C) Both (A) and (B)
Q69. An alcohol on oxidation gives a ketone. The alcohol is:
(A) Ethanol
(B) Methanol
(C) 2-Propanol
(D) 2-Methyl-1-propanol
Answer: (C) 2-Propanol
Q70. Which ether cannot be prepared by Williamson synthesis?
(A) CH₃CH₂OCH₃
(B) CH₃OC₆H₅
(C) C₆H₅OCH₃
(D) (CH₃)₃COCH₃
Answer: (D) (CH₃)₃COCH₃
Q71. The major product when phenol is treated with excess Br₂ in water is:
(A) o-Bromophenol
(B) p-Bromophenol
(C) 2,4,6-Tribromophenol
(D) m-Bromophenol
Answer: (C) 2,4,6-Tribromophenol
Q72. Which of the following reagents can distinguish ethanol from methanol?
(A) Tollen’s reagent
(B) Lucas reagent
(C) Iodoform reagent
(D) Schiff’s reagent
Answer: (C) Iodoform reagent
Q73. Phenol reacts with CO₂ and NaOH under heat to form:
(A) o-Hydroxybenzaldehyde
(B) Salicylic acid
(C) o-Nitrophenol
(D) Phenyl acetate
Answer: (B) Salicylic acid
Q74. Arrange the following in increasing order of acid strength:
Phenol, p-Nitrophenol, p-Cresol
(A) p-Cresol < Phenol < p-Nitrophenol
(B) Phenol < p-Cresol < p-Nitrophenol
(C) p-Nitrophenol < Phenol < p-Cresol
(D) p-Cresol < p-Nitrophenol < Phenol
Answer: (A) p-Cresol < Phenol < p-Nitrophenol
Q75. Alcohols generally have higher boiling points than alkanes due to:
(A) Strong dipole-dipole interaction
(B) Resonance
(C) Hydrogen bonding
(D) Van der Waals forces
Answer: (C) Hydrogen bonding
Q76. Which of the following is most reactive towards dehydration?
(A) Ethanol
(B) 1-Propanol
(C) 2-Propanol
(D) tert-Butyl alcohol
Answer: (D) tert-Butyl alcohol
Q77. The electrophile in Reimer–Tiemann reaction is:
(A) –OH
(B) CHCl₃
(C) Dichlorocarbene (:CCl₂)
(D) Cl⁻
Answer: (C) Dichlorocarbene (:CCl₂)
Q78. The presence of which group increases the acidity of phenol?
(A) –CH₃
(B) –OCH₃
(C) –NO₂
(D) –NH₂
Answer: (C) –NO₂
Q79. Which alcohol gives fastest reaction with Lucas reagent?
(A) Ethanol
(B) 1-Butanol
(C) 2-Butanol
(D) tert-Butanol
Answer: (D) tert-Butanol
Q80. Phenol on reaction with Zn gives:
(A) Benzene
(B) Toluene
(C) Benzoic acid
(D) Aniline
Answer: (A) Benzene
Q81. Which of the following has the least acidic proton?
(A) Phenol
(B) Water
(C) Ethanol
(D) Acetic acid
Answer: (C) Ethanol
Q82. What is the hybridisation of carbon in methanol?
(A) sp³
(B) sp²
(C) sp
(D) None of these
Answer: (A) sp³
Q83. The reaction of CH₃CH₂OH with SOCl₂ gives:
(A) CH₃CH₂Cl
(B) CH₃CH₂OH
(C) CH₃CH₂SH
(D) CH₃CHO
Answer: (A) CH₃CH₂Cl
Q84. Which compound will give a positive FeCl₃ test?
(A) Methanol
(B) Ethanol
(C) Phenol
(D) Ether
Answer: (C) Phenol
Q85. Williamson synthesis fails for:
(A) CH₃CH₂ONa + CH₃CH₂Br
(B) C₆H₅ONa + CH₃Br
(C) CH₃ONa + C₆H₅Br
(D) CH₃CH₂ONa + CH₃CH₂Br
Answer: (C) CH₃ONa + C₆H₅Br
Q86. In Kolbe’s reaction, the phenoxide ion acts as:
(A) Nucleophile
(B) Electrophile
(C) Leaving group
(D) Acid
Answer: (A) Nucleophile
Q87. Which of the following gives iodoform test?
(A) Methanol
(B) Ethanol
(C) 2-Propanol
(D) Both (B) and (C)
Answer: (D) Both (B) and (C)
Q88. Heating of CH₃CH₂OH with concentrated H₂SO₄ gives:
(A) CH₂=CH₂
(B) CH₃CH₂Cl
(C) CH₃CHO
(D) CH₃CH₂HSO₄
Answer: (A) CH₂=CH₂
Q89. Which of the following reacts fastest in acid-catalysed dehydration?
(A) 1-Butanol
(B) 2-Butanol
(C) tert-Butanol
(D) Methanol
Answer: (C) tert-Butanol
Q90. The bond angle in the C–O–C group in ethers is:
(A) 180°
(B) 109.5°
(C) 104.5°
(D) 120°
Answer: (C) 104.5°
Q91. Which of the following is not an example of alcohol?
(A) Glycerol
(B) Methanol
(C) Isopropanol
(D) Acetone
Answer: (D) Acetone
Q92. Sodium reacts violently with:
(A) Methanol
(B) Diethyl ether
(C) Benzene
(D) Acetone
Answer: (A) Methanol
Q93. Phenol shows higher acidity than ethanol due to:
(A) High molecular mass
(B) Resonance stabilization of phenoxide
(C) Presence of benzene ring
(D) Inductive effect of –OH
Answer: (B) Resonance stabilization of phenoxide
Q94. The correct order of solubility in water is:
(A) Phenol > Methanol > Ethanol
(B) Methanol > Ethanol > Phenol
(C) Ethanol > Methanol > Phenol
(D) Phenol > Ethanol > Methanol
Answer: (B) Methanol > Ethanol > Phenol
Q95. Boiling point order among the following is:
(A) Diethyl ether < Methanol < Water
(B) Water < Methanol < Diethyl ether
(C) Methanol < Diethyl ether < Water
(D) Diethyl ether < Water < Methanol
Answer: (A) Diethyl ether < Methanol < Water
Q96. The molecular formula of an ether is C₄H₁₀O. Which of the following is correct?
(A) CH₃CH₂CH₂OCH₃
(B) CH₃CH₂CH₂CH₂OH
(C) CH₃CH₂OH
(D) CH₃CH₂CH₂CH₃
Answer: (A) CH₃CH₂CH₂OCH₃
Q97. In the reaction of phenol with neutral FeCl₃, the colored complex formed is:
(A) Green
(B) Purple
(C) Orange
(D) Red
Answer: (B) Purple
Q98. The number of hydrogen atoms replaced when phenol reacts with Na is:
(A) 1
(B) 2
(C) 3
(D) None
Answer: (A) 1
Q99. Which among the following does not undergo dehydration easily?
(A) 2-Propanol
(B) Ethanol
(C) Methanol
(D) 2-Methyl-2-propanol
Answer: (C) Methanol
Q100. Heating phenol with CHCl₃ and aq. NaOH forms a compound having:
(A) CHO group
(B) COOH group
(C) –NO₂ group
(D) –OH group
Answer: (A) CHO group
————————————————————————————————————————————————————————————————————————————
MISCONCEPTIONS “ALERTS”

————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN
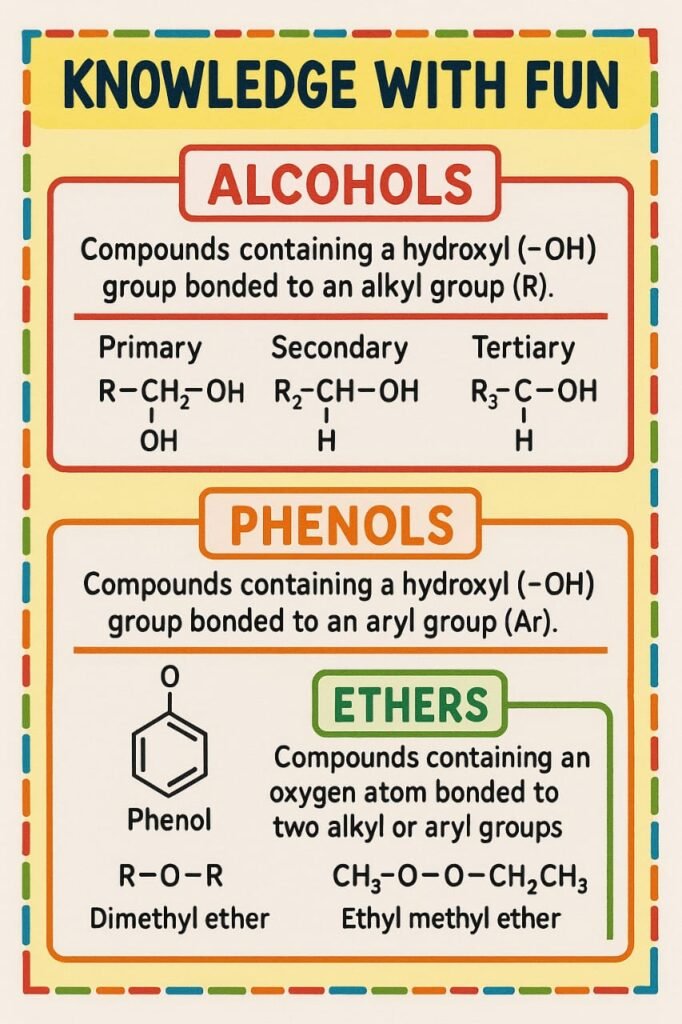
————————————————————————————————————————————————————————————————————————————
MNEMONICS
———————————————————————————————————————————————————————————————————————————–
MINDMAPS