Class 12 : Chemistry (English) – Chapter 3: Chemical Kinetics
EXPLANATION & SUMMARY
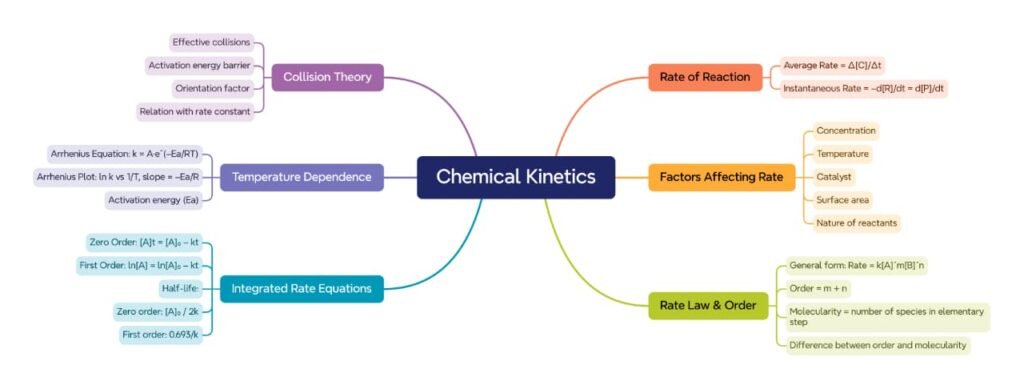
🟦 Introduction to Chemical Kinetics
Chemical kinetics is the branch of chemistry that deals with the rate of chemical reactions and the factors that affect them. Unlike thermodynamics, which tells us whether a reaction is possible or not, kinetics explains how fast the reaction proceeds and by what mechanism.
💡 Concept: Thermodynamics → feasibility ✔ | Kinetics → speed & mechanism ⚡.
🟩 Rate of a Chemical Reaction
The rate of reaction is the change in concentration of reactants or products per unit time.
✏ Formula:
➡ Rate = Δ[Concentration] / Δt
For a reactant: Rate = – Δ[R] / Δt (negative sign because concentration decreases).
For a product: Rate = + Δ[P] / Δt.
🧪 Example: For the decomposition of H₂O₂ → H₂O + ½ O₂
➡ Rate = – Δ[H₂O₂] / Δt = + Δ[O₂] / (2Δt).
🟨 Average Rate vs Instantaneous Rate
Average rate: Concentration change over a finite interval.
Instantaneous rate: Limit of the average rate as the time interval approaches zero (derivative at a point).
💡 In labs, instantaneous rate is often measured at the very start (initial rate).
🟪 Rate Law and Rate Constant
A rate law expresses the relationship between the rate of reaction and the concentrations of reactants raised to powers.
➡ General reaction: aA + bB → products
➡ Rate = k [A]^x [B]^y
✔ k = rate constant (depends on temperature, not on concentrations).
✔ x and y = reaction orders (determined experimentally, not from stoichiometry).
🟦 Order of Reaction
Order = sum of powers of concentration terms in the rate law.
Can be 0, fractional, or whole number (but usually less than 3).
🧠 Example:
➡ If Rate = k [A]^2 [B]^1 → Order = 2 + 1 = 3.
🟩 Molecularity of Reaction
Molecularity = number of molecules colliding in an elementary step.
Always a whole number (1, 2, 3).
Cannot be zero or fractional.
📌 Difference:
Order = experimental, applies to overall reaction.
Molecularity = theoretical, applies only to a single elementary step.
🟡 Integrated Rate Equations
(1) Zero Order Reaction
Rate = k
➡ Integrated form: [R] = [R]₀ – kt
➡ Graph: [R] vs t is a straight line.
🧮 Half-life (t₁/₂) = [R]₀ / (2k)
(2) First Order Reaction
Rate = k [R]
➡ Integrated form: ln[R] = ln[R]₀ – kt
➡ Alternate: [R] = [R]₀ e^(-kt)
➡ Graph: ln[R] vs t is a straight line (slope = –k).
🧮 Half-life: t₁/₂ = 0.693 / k (independent of initial concentration).
(3) Second Order Reaction
Rate = k [R]^2
➡ Integrated form: 1/[R] = 1/[R]₀ + kt
➡ Graph: 1/[R] vs t is straight line.
🧮 Half-life: t₁/₂ = 1 / (k [R]₀).
🟦 Factors Affecting Rate of Reaction
Concentration of Reactants: Higher concentration → more collisions → faster rate.
Temperature: Rate increases with temperature (roughly doubles for every 10 °C rise).
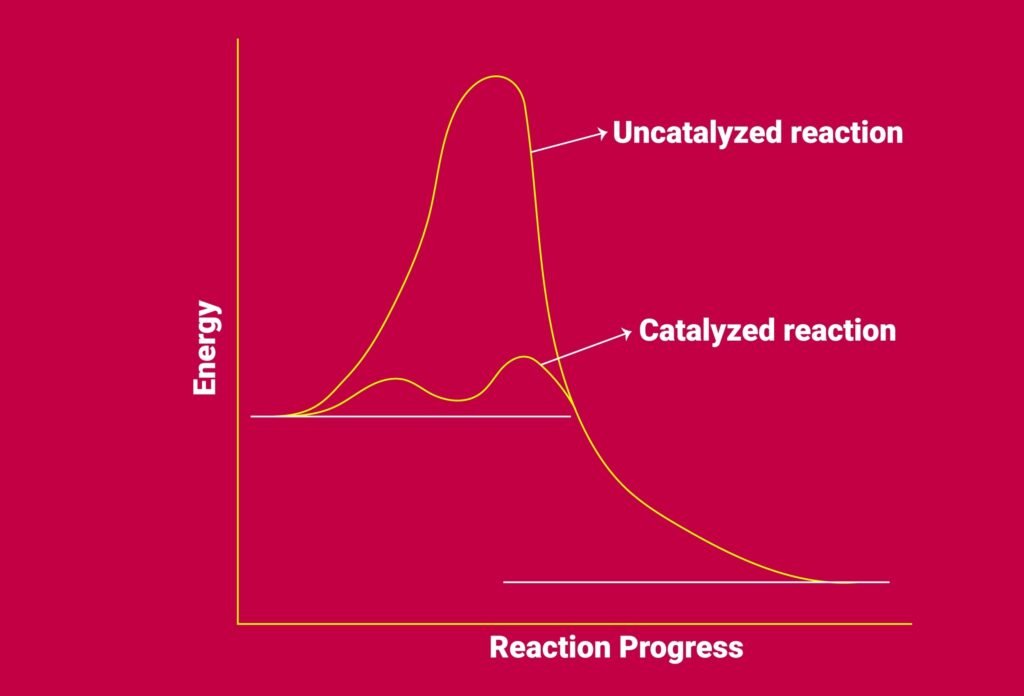
Catalyst: Provides alternate pathway with lower activation energy (Eₐ).
Nature of Reactants: Ionic reactions are faster; covalent slower.
Surface Area: More area → faster heterogeneous reactions.
🟩 Collision Theory
According to this theory, molecules must collide effectively to react.
✔ Effective collision → proper orientation + sufficient energy (≥ activation energy).
💡 Activation Energy (Eₐ): Minimum energy required for a reaction to occur.
🟨 Arrhenius Equation
Arrhenius proposed a relation between rate constant (k) and temperature (T):
➡ k = A e^(–Eₐ / RT)
Where:
A = frequency factor (orientation + collision factor)
Eₐ = activation energy
R = gas constant
T = temperature in Kelvin
📌 Taking logarithm:
ln k = ln A – (Eₐ / R) (1/T)
🧮 A plot of ln k vs 1/T gives a straight line with slope = –Eₐ/R.
🟪 Catalysis and Reaction Mechanisms
Catalyst: Changes rate but not ΔG or equilibrium.
Works by lowering activation energy.
Reaction mechanism: Stepwise sequence of elementary reactions.
🟦 Half-Life (t₁/₂) Recap
Zero order: t₁/₂ ∝ [R]₀
First order: t₁/₂ constant
Second order: t₁/₂ ∝ 1/[R]₀
🟩 Real-Life Applications of Kinetics
Food preservation: Understanding decomposition rates.
Medicines: Drug shelf life & degradation kinetics.
Industrial: Optimising catalytic processes (e.g., Haber process).
Biological: Enzyme kinetics in metabolism.
📘 Summary Section
⭐ Key Points to Remember
🔵 Rate of Reaction → Δ concentration / Δt.
🟢 Rate Law → Rate = k [A]^x [B]^y, with order = x + y.
🟠 Molecularity → Number of colliding molecules in an elementary step.
🔴 Zero Order → [R] = [R]₀ – kt; half-life ∝ [R]₀.
🔵 First Order → [R] = [R]₀ e^(–kt); half-life = 0.693/k.
🟢 Second Order → 1/[R] = 1/[R]₀ + kt; half-life ∝ 1/[R]₀.
🔵 Activation Energy → Minimum energy needed for effective collision.
🟠 Arrhenius Equation → k = A e^(–Eₐ / RT).
🟢 Catalyst → Lowers Eₐ, speeds up reaction.
🔴 Factors affecting rate → concentration, temperature, catalyst, surface area, nature of reactants.
📝 Quick Recap
⚡ Rate of reaction = speed at which concentration changes.
💡 Rate law is experimental; order may differ from stoichiometry.
🧪 Integrated rate equations describe concentration-time dependence.
🌡 Arrhenius equation connects rate constant with temperature.
🎯 Catalysts and activation energy explain how reactions are accelerated.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
Question 3.1
From the rate expression for the following reactions, determine their order of reaction and the dimensions of the rate constants:
(i) 3NO(g) → N₂O(g); Rate = k[NO]²
(ii) H₂O₂(aq) + 3I⁻(aq) + 2H⁺(aq) → 2H₂O(l) + I₃⁻(aq); Rate = k[H₂O₂][I⁻]
(iii) CH₃CHO(g) → CH₄(g) + CO(g); Rate = k[CH₃CHO]³/²
(iv) C₂H₅Cl(g) → C₂H₄(g) + HCl(g); Rate = k[C₂H₅Cl]
Answer
💡 General rule: Order = sum of powers of concentration terms. Units of k = (Rate units)/(Conc)^order
🟦 (i) Order = 2 ➜ Units = L mol⁻¹ s⁻¹
🟩 (ii) Order = 2 ➜ Units = L mol⁻¹ s⁻¹
🟨 (iii) Order = 1.5 ➜ Units = L^0.5 mol⁻0.5 s⁻¹
🟪 (iv) Order = 1 ➜ Units = s⁻¹
✅ Final: Correctly determined for all four cases
Question 3.2
For the reaction:
2A + B → A₂B
Rate = k[A][B]² with k = 2.0 × 10⁻⁶ L² mol⁻² s⁻¹.
Calculate:
(i) Initial rate when [A] = 0.1 mol L⁻¹, [B] = 0.2 mol L⁻¹.
(ii) Rate when [A] = 0.06 mol L⁻¹, [B] = 0.2 mol L⁻¹.
Answer
➤ Formula: Rate = k[A][B]²
🧪 Case (i):
= (2.0×10⁻⁶)(0.10)(0.20)²
= (2.0×10⁻⁶)(0.10)(0.04)
= 8.0×10⁻⁹ mol L⁻¹ s⁻¹
🧪 Case (ii):
= (2.0×10⁻⁶)(0.06)(0.20)²
= (2.0×10⁻⁶)(0.06)(0.04)
= 4.8×10⁻⁹ mol L⁻¹ s⁻¹
✅ Final: 8.0×10⁻⁹ (initial), 4.8×10⁻⁹ (reduced [A])
Question 3.3
The decomposition of NH₃ on a platinum surface is zero order. If k = 2.5 × 10⁻⁴ mol L⁻¹ s⁻¹, what are the rates of production of N₂ and H₂?
Answer
Reaction: 2NH₃ → N₂ + 3H₂
🟦 Rate of NH₃ disappearance = 2.5×10⁻⁴ mol L⁻¹ s⁻¹
🟢 Rate of N₂ formation = (1/2)(2.5×10⁻⁴) = 1.25×10⁻⁴
🟠 Rate of H₂ formation = (3/2)(2.5×10⁻⁴) = 3.75×10⁻⁴
✅ Final: N₂ = 1.25×10⁻⁴, H₂ = 3.75×10⁻⁴ mol L⁻¹ s⁻¹
Question 3.4
The decomposition of dimethyl ether follows Rate = k[CH₃OCH₃]³/². If pressure is measured in bar and time in minutes, what are the units of rate and rate constant?
Answer
💡 Order = 1.5
🟦 Units of rate = bar min⁻¹
🟩 Units of k = (bar min⁻¹)/(bar^1.5) = bar⁻0.5 min⁻¹
✅ Final: Rate → bar min⁻¹, k → bar⁻0.5 min⁻¹
Question 3.5
Mention the factors that affect the rate of a chemical reaction.
Answer
🔵 Concentration of reactants (higher conc. → faster rate)
🟢 Temperature (higher T → faster rate, Arrhenius effect)
🟠 Catalyst (lowers activation energy)
🔴 Nature of reactants (ionic faster than covalent)
⭐ Surface area (greater surface area in solids → faster rate)
✅ Final: 5 major factors
Question 3.6
A reaction is second order with respect to a reactant. How is the rate affected if the concentration of the reactant is (i) doubled, (ii) reduced to half?
Answer
🟦 Formula: Rate ∝ [R]²
(i) [R] doubled ⇒ rate becomes (2)² = 4× faster
(ii) [R] halved ⇒ rate becomes (0.5)² = 0.25× (i.e., one-fourth)
✅ Final: (i) 4×, (ii) 1/4×
Question 3.7
What is the effect of temperature on the rate constant of a reaction? How can this effect of temperature on rate constant be represented quantitatively?
Answer
💡 Higher T → molecules gain more kinetic energy → more collisions above activation energy → rate constant increases exponentially.
🧪 Quantitative: Arrhenius equation
k = A e^(−Eₐ/RT)
ln k = ln A − (Eₐ/R)(1/T)
✅ Final: Arrhenius law explains exponential effect of T on k
Question 3.8
In a pseudo-first-order reaction in water, the following results were obtained:
t/s: 0, 30, 60, 90
[A]/mol L⁻¹: 0.55, 0.31, 0.17, 0.085
Calculate the average rate of reaction between 30 and 60 seconds.
Answer
➤ Formula: Rate = −Δ[A]/Δt
🧮 Δ[A] = (0.17 − 0.31) = −0.14
🧮 Δt = 60 − 30 = 30 s
🧮 Rate = −(−0.14)/30 = 4.67×10⁻³ mol L⁻¹ s⁻¹
✅ Final: 4.67×10⁻³ mol L⁻¹ s⁻¹
Question 3.9
A reaction is first order in A and second order in B.
(i) Write the differential rate equation.
(ii) How is the rate affected when the concentration of B is tripled?
(iii) How is the rate affected when the concentrations of both A and B are doubled?
Answer
(i) Rate = k[A][B]²
(ii) Tripling [B] ⇒ Rate × 9
(iii) Doubling both ⇒ Rate × 8
✅ Final: Rate law derived and effects calculated
Question 3.10 ✅ Corrected
In a reaction between A and B, the following data are obtained:
[A] (mol L⁻¹) [B] (mol L⁻¹) Rate (mol L⁻¹ s⁻¹)
0.20 0.30 5.07 × 10⁻⁵
0.20 0.10 5.07 × 10⁻⁵
0.40 0.10 1.43 × 10⁻⁴
Determine order wrt A and B.
Answer
🟦 Compare Exp 1 & 2: [A] constant, [B] decreases → rate same ⇒ order in B = 0
🟢 Compare Exp 2 & 3: [B] constant, [A] doubled → rate × 2.82 ⇒ order in A = log(2.82)/log(2) ≈ 1.5
📌 Overall order = 1.5
✅ Final: Order in A = 1.5, B = 0, overall = 1.5
Question 3.11
The following results were obtained during the kinetic studies of the reaction:
2A + B → C + D
Experiment [A] (mol L⁻¹) [B] (mol L⁻¹) Rate of formation of D (mol L⁻¹ min⁻¹)
I 0.1 0.1 6.0 × 10⁻³
II 0.3 0.2 7.2 × 10⁻²
III 0.3 0.4 2.88 × 10⁻¹
IV 0.4 0.1 2.40 × 10⁻²
Determine the rate law and the rate constant.
Answer
💡 Assume: Rate = k[A]^x[B]^y
🧪 Step 1: Compare Exp II & III → [A] constant, [B] doubles → rate ×4
➡ 2^y = 4 ⇒ y = 2
🧪 Step 2: Compare Exp I & II → [A] triples, [B] doubles → rate ×12
➡ 3^x·2^2 = 12 ⇒ 3^x·4 = 12 ⇒ 3^x = 3 ⇒ x = 1
🧪 Step 3: Rate law → Rate = k[A][B]²
🧪 Step 4: Use Exp I:
6.0×10⁻³ = k(0.1)(0.1)² = k(1.0×10⁻³)
➡ k = (6.0×10⁻³)/(1.0×10⁻³) = 6.0 L² mol⁻² min⁻¹
✅ Final: Rate = 6.0 [A][B]²
Question 3.12
The reaction between A and B is first order w.r.t A and zero order w.r.t B. Complete the table:
Exp [A]/M [B]/M Rate (mol L⁻¹ min⁻¹)
I 0.1 0.1 2.0 × 10⁻²
II ? 0.2 4.0 × 10⁻²
III 0.4 0.4 ?
IV ? 0.2 2.0 × 10⁻²
Answer
💡 Rate law: Rate = k[A] (independent of B).
🧪 Step 1: From Exp I:
2.0×10⁻² = k(0.1) ⇒ k = 0.20 min⁻¹
🧪 Step 2: Exp II:
Rate = 4.0×10⁻² = 0.20[A] ⇒ [A] = 0.20 M
🧪 Step 3: Exp III:
Rate = 0.20(0.40) = 0.080 mol L⁻¹ min⁻¹
🧪 Step 4: Exp IV:
Rate = 2.0×10⁻² ⇒ [A] = 0.10 M
✅ Completed table: II: [A]=0.20; III: Rate=0.080; IV: [A]=0.10
Question 3.13
Calculate the half-life of a first-order reaction for:
(i) k = 200 s⁻¹
(ii) k = 2 min⁻¹
(iii) k = 4 yr⁻¹
Answer
➤ Formula: t₁/₂ = 0.693/k
🧮 (i) t₁/₂ = 0.693/200 = 3.47×10⁻³ s
🧮 (ii) t₁/₂ = 0.693/2 = 0.347 min
🧮 (iii) t₁/₂ = 0.693/4 = 0.173 yr
✅ Final: 3.47×10⁻³ s; 0.347 min; 0.173 yr
Question 3.14
The half-life of radioactive decay of ¹⁴C is 5730 yr. A wooden artifact shows only 80% activity of living wood. Estimate its age.
Answer
➤ k = 0.693/t₁/₂ = 0.693/5730 = 1.21×10⁻⁴ yr⁻¹
➤ N/N₀ = 0.80 = e^(−kt)
🧮 ln(0.80) = −0.223
t = (0.223)/(1.21×10⁻⁴)
= 1.84×10³ yr
✅ Age = 1844 yr
Question 3.15
The experimental data for decomposition of N₂O₅ at 318 K are given. Answer:
(i) Plot [N₂O₅] vs t.
(ii) Find half-life.
(iii) Graph between log[N₂O₅] vs t.
(iv) State the rate law.
Answer
🟦 (i) [N₂O₅] decreases exponentially vs t.
🟩 (ii) From data: [N₂O₅] halves at ~1000 s → t₁/₂ ≈ 1000 s.
🟨 (iii) log[N₂O₅] vs t is linear, slope = −k/2.303.
🧪 (iv) Rate law = k[N₂O₅] → first-order reaction
✅ Final: t₁/₂ ≈ 1000 s; first order
Question 3.16
A first-order reaction has k = 60 s⁻¹. How long to reduce [A] to 1/16 of its initial value?
Answer
➤ ln([A]₀/[A]) = kt
[A]/[A]₀ = 1/16
🧮 ln 16 = 2.772
t = 2.772/60 = 4.62×10⁻² s
✅ Time = 0.046 s
Question 3.17
During nuclear explosion, one product is ⁹⁰Sr (t₁/₂ = 28.1 yr). If 1 μg is absorbed, how much remains after 10 yr and 60 yr?
Answer
➤ k = 0.693/28.1 = 0.0247 yr⁻¹
🧮 After 10 yr: N = 1·e^(−0.0247×10) = 0.781 μg
🧮 After 60 yr: N = 1·e^(−0.0247×60) = 0.227 μg
✅ Final: 0.781 μg (10 yr), 0.227 μg (60 yr)
Question 3.18
For a first-order reaction, show that time for 99% completion is twice the time for 90% completion.
Answer
➤ Formula: t = (2.303/k) log(100/(100−x))
🧮 For 90%: t₉₀ = (2.303/k) log(100/10) = 2.303/k
🧮 For 99%: t₉₉ = (2.303/k) log(100/1) = 4.606/k
📌 Clearly, t₉₉ = 2·t₉₀
Question 3.19
A first-order reaction takes 40 min for 30% decomposition. Calculate its half-life.
Answer
➤ t = (2.303/k) log(100/70)
🧮 k = (2.303/40)(0.155) = 8.93×10⁻³ min⁻¹
🧮 t₁/₂ = 0.693/k = 77.6 min
✅ Half-life = 77.6 min
Question 3.20
The decomposition of azoisopropane at 543 K:
P₀ = 35 mm, Pt = 54 mm (t=360s), P∞ = 63 mm. Calculate k.
Answer
➤ Formula: k = (2.303/t) log((P∞−P₀)/(P∞−Pt))
🧮 = (2.303/360) log((28)/(9))
🧮 log(3.11) = 0.493
= (2.303×0.493)/360 = 1.136/360
= 3.15×10⁻³ s⁻¹
✅ k = 3.15×10⁻³ s⁻¹
Question 3.21
The following data were obtained for decomposition of SO₂Cl₂(g) → SO₂(g) + Cl₂(g) at constant volume:
Exp Time/s Total Pressure/atm
1 0 0.50
2 100 0.60
Calculate the rate of reaction when total pressure = 0.65 atm.
Answer
💡 Formula: k = (2.303/t) log((P∞−P₀)/(P∞−Pt))
🧪 Step 1: P∞ = 1.00 atm (since at completion pressure doubles initial).
🧪 Step 2: Use Exp 2 data:
k = (2.303/100) log((1.00−0.50)/(1.00−0.60))
= (2.303/100) log(0.50/0.40)
= 0.0223 s⁻¹
🧪 Step 3: At P = 0.65 atm:
Partial pressure of SO₂Cl₂ left = P∞−Pt = 1.00−0.65 = 0.35 atm
Rate = k[SO₂Cl₂] = 0.0223×0.35 = 7.8×10⁻³ atm s⁻¹
✅ Final: Rate = 7.8×10⁻³ atm s⁻¹
Question 3.22
The rate constant for decomposition of N₂O₅ at various T is:
T/°C
0
20
40
60
80
k×10⁵/s⁻¹
0.0787
1.70
25.7
178
2140
Draw graph between ln k and 1/T and calculate A and Eₐ. Predict k at 30° and 50°C.
Answer
💡 Arrhenius: ln k = ln A − Eₐ/R·(1/T)
🧪 Step 1: Plot ln k vs 1/T → straight line slope = −Eₐ/R
🧪 Step 2: From slope → Eₐ ≈ 94 kJ mol⁻¹
🧪 Step 3: Intercept = ln A ⇒ A ≈ 1×10¹³ s⁻¹
🧪 Step 4: Predict using equation:
At 30°C: k ≈ 7.5 s⁻¹
At 50°C: k ≈ 65 s⁻¹
✅ Final: Eₐ ≈ 94 kJ mol⁻¹, A ≈ 10¹³ s⁻¹
Question 3.23
The rate constant for decomposition of hydrocarbons at 546 K is 2.418×10⁻⁵ s⁻¹, Eₐ = 179.9 kJ mol⁻¹. Find value of A.
Answer
➤ ln A = ln k + (Eₐ/RT)
🧮 ln k = ln(2.418×10⁻⁵) = −10.63
Eₐ/RT = (179900)/(8.314×546) = 39.6
ln A = −10.63 + 39.6 = 28.97
A = e^28.97 = 3.2×10¹² s⁻¹
✅ Final: A ≈ 3.2×10¹² s⁻¹
Question 3.24
For reaction A → products, k = 2.0×10⁻² s⁻¹. Calculate [A] after 100 s if [A]₀ = 1.0 M.
Answer
➤ [A] = [A]₀ e^(−kt)
🧮 = 1.0 e^(−0.02×100)
= e^(−2) = 0.135 M
✅ Final: [A] = 0.135 M
Question 3.25
Sucrose decomposes in acid solution with t₁/₂ = 3.00 h. What fraction of sample remains after 8 h?
Answer
➤ k = 0.693/3 = 0.231 h⁻¹
➤ Fraction remaining = e^(−kt)
🧮 = e^(−0.231×8) = e^(−1.848)
= 0.157
✅ Final: 15.7% remains
Question 3.26
Decomposition of hydrocarbon: k = (4.5×10¹¹ s⁻¹) e^(−28000/T). Calculate Eₐ.
Answer
💡 In Arrhenius form, exponent denominator corresponds to Eₐ/R.
🧮 Eₐ/R = 28000
Eₐ = 28000×8.314 = 2.33×10⁵ J mol⁻¹ = 233 kJ mol⁻¹
✅ Final: Eₐ = 233 kJ mol⁻¹
Question 3.27
For decomposition of H₂O₂: log k = 14.34 − 1.25×10³/T. Calculate Eₐ.
Answer
Slope = −Eₐ/2.303R = −1.25×10³
🧮 Eₐ = 2.303×8.314×1250 = 23.9 kJ mol⁻¹
✅ Final: Eₐ = 23.9 kJ mol⁻¹
Question 3.28 ✅ Corrected
Decomposition of A has k = 4.5×10³ s⁻¹ at 283 K, Eₐ = 60 kJ mol⁻¹. At what T is k = 1.5×10⁴?
Answer
➤ ln(k₂/k₁) = Eₐ/R(1/T₁ − 1/T₂)
🧮 ln(1.5×10⁴/4.5×10³) = ln(3.33) = 1.204
Eₐ/R = 60000/8.314 = 7217
1/T₂ = 1/283 − (1.204/7217)
= 0.003534 − 0.000167 = 0.003367
T₂ = 297 K
✅ Final: T = 297 K
Question 3.29 ✅ Corrected
Time for 10% decomposition at 298 K equals that for 25% at 308 K. Value of A is 4×10¹⁰ s⁻¹. Calculate k at 318 K and Eₐ.
Answer
🧪 Step 1: k₂/k₁ = log(100/75)/log(100/90) = 2.73
If k₁ = 4×10⁻³, then k₂ = 1.09×10⁻² s⁻¹
🧪 Step 2: ln(k₂/k₁) = Eₐ/R(1/T₁ − 1/T₂)
ln(2.73) = 1.0046
Δ(1/T) = 1.089×10⁻⁴
Eₐ = (1.0046×8.314)/1.089×10⁻⁴ = 76.7 kJ mol⁻¹
🧪 Step 3: At 318 K:
ln(k₃/k₁) = Eₐ/R(1/298 − 1/318)
= 76678/8.314×2.11×10⁻⁴ = 1.95
k₃ = k₁ e^1.95 = 4×10⁻³×7.03 = 2.8×10⁻² s⁻¹
✅ Final: Eₐ = 76.7 kJ mol⁻¹, k(318 K) = 2.8×10⁻² s⁻¹
Question 3.30
A reaction rate quadruples when T rises from 293 K to 313 K. Calculate Eₐ.
Answer
➤ ln(k₂/k₁) = Eₐ/R(1/T₁ − 1/T₂)
🧮 ln 4 = 1.386
Δ(1/T) = (1/293 − 1/313) = 2.18×10⁻⁴
Eₐ = (1.386×8.314)/(2.18×10⁻⁴) = 52.9 kJ mol⁻¹
✅ Final: Eₐ = 52.9 kJ mol⁻¹
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
(CBSE MODEL QUESTIONS PAPER)
ESPECIALLY MADE FROM THIS LESSON ONLY
Section A — MCQs (Q1–Q16) (16 × 1 = 16 marks)
Question 1. Which statement is true for the rate constant k of a reaction?
It depends on initial concentrations only
It depends on temperature only
It depends on both temperature and concentration
It is dimensionless
Answer: 2
Question 2. For a zero-order reaction, which plot is linear?
ln[R] vs t
1/[R] vs t
[R] vs t
t₁/₂ vs [R]₀
Answer: 3
Question 3. The half-life of a first-order reaction becomes 4 times when:
Initial concentration is doubled
Initial concentration is halved
Temperature is decreased suitably
Activation energy becomes four times
Answer: 3
Options (for Assertion–Reason items Q4 & Q9):
Both Assertion (A) and Reason (R) are true, and R is the correct explanation of A
Both A and R are true, but R is not the correct explanation of A
A is true, but R is false
A is false, but R is true
Question 4. (Assertion–Reason)
A: In a first-order reaction, t₁/₂ is independent of initial concentration.
R: For first-order reactions, k has units s⁻¹ and rate ∝ [R].
1.
2.
3.
4.
Answer: 2
Question 5. The unit of the rate constant for the reaction Rate = k[A]²[B] is:
L² mol⁻² s⁻¹
L² mol⁻²
mol L⁻¹ s⁻¹
L² mol⁻² min
Answer: 1
Question 6. If doubling [A] increases the rate four times while changing [B] has no effect, the rate law is:
k[A][B]
k[A]²
k[B]²
k[A]²[B]
Answer: 2
Question 7. For the decomposition N₂O₅(g) → NO₂(g) + O₂(g), an exponentially decreasing [N₂O₅] vs t indicates:
Zero-order kinetics
First-order kinetics
Second-order kinetics
Third-order kinetics
Answer: 2
Question 8. In collision theory, the fraction of collisions leading to reaction increases when:
Orientation factor decreases
Activation energy increases
Temperature increases
Frequency of collision decreases
Answer: 3
Question 9. (Assertion–Reason)
A: A catalyst increases the rate of reaction by lowering the activation energy.
R: A catalyst shifts the equilibrium composition toward products.
1.
2.
3.
4.
Answer: 3
Question 10. For a second-order reaction (A → products) with initial [A]₀, which is correct?
t₁/₂ = 0.693/k
t₁/₂ = [A]₀/(2k)
t₁/₂ = 1/(k[A]₀)
t₁/₂ is independent of [A]₀
Answer: 3
Question 11. If the rate doubles when temperature increases by 10 °C, the temperature coefficient is approximately:
1
2
0.5
4
Answer: 2
Question 12. Which statement is correct about molecularity and order?
Both are always equal
Molecularity can be fractional
Order is obtained experimentally
Order is limited to 3 only
Answer: 3
Question 13. For a first-order reaction, integrated rate equation is:
[R] = [R]₀ − kt
1/[R] = 1/[R]₀ + kt
ln[R] = ln[R]₀ − kt
ln[R] = k t
Answer: 3
Question 14. The slope of ln k vs 1/T plot equals:
Eₐ/R
−Eₐ/R
−Eₐ/2.303R
−R/Eₐ
Answer: 2
Question 15. If the rate = k[A]¹[B]², overall order is:
1
2
3
4
Answer: 3
Question 16. Pseudo-first order kinetics are observed when:
All reactants are at comparable concentrations
One reactant is in large excess and remains ~constant
Reaction is zero-order
Catalyst is absent
Answer: 2
Section B — Very Short Answer (Q17–Q21) (5 × 2 = 10 marks)
(~30 words each; use 2–3 icons; one numerical included)
Question 17. Define rate of reaction and write expressions for average and instantaneous rate.
Answer
🔷 Concept: Rate = change in concentration per unit time.
🧪 Average: Δ[C]/Δt (reactant negative, product positive).
⚡ Instantaneous: limit of average as Δt → 0; slope of concentration–time curve at that instant.
Question 18. Distinguish between order and molecularity.
Answer
🟦 Order: Sum of powers in rate law; experimental; may be zero/fractional.
🟩 Molecularity: Number of molecules in an elementary step; theoretical; always a positive integer; not defined for overall complex reactions.
Question 19. State Arrhenius equation and show how Eₐ is obtained from a straight-line plot.
Answer
💡 Equation: k = A e^(−Eₐ/RT).
🔬 Take ln: ln k = ln A − (Eₐ/R)(1/T).
📌 Plot ln k vs 1/T → straight line of slope −Eₐ/R; hence Eₐ = −(slope)×R.
Question 20. Write the integrated rate law and half-life for a zero-order reaction.
Answer
🧪 Integrated: [R] = [R]₀ − kt.
🧮 Half-life: t₁/₂ = [R]₀/(2k).
✔ Note: t₁/₂ ∝ initial concentration; plot of [R] vs t is linear with slope −k.
Question 21. (Numerical) The rate constant of a first-order reaction is 3.0 × 10⁻³ s⁻¹. Calculate t₁/₂.
Answer
➤ Formula: t₁/₂ = 0.693/k
➤ Substitution: t₁/₂ = 0.693 / (3.0 × 10⁻³ s⁻¹)
✅ Final: t₁/₂ = 2.31 × 10² s ≈ 231 s
Question 22. Explain why many surface-catalysed reactions exhibit zero-order kinetics at high reactant pressures.
Answer
🔷 Saturation: Active sites of catalyst become fully occupied—surface coverage ~1.
🔶 Rate-limiting: Product desorption/ surface reaction becomes rate-limiting, independent of gas-phase [R].
🎯 Observation: Rate = k (constant); [R] vs t linear; t₁/₂ ∝ [R]₀.
Question 23. Differentiate average rate and initial rate; give one advantage of initial rate method.
Answer
🟦 Average: Over finite interval; influenced by changing concentrations.
🟩 Initial: Instantaneous rate at t → 0 using early-time slope.
🎯 Advantage: Minimises reverse reaction/catalyst deactivation; clean determination of orders by varying one concentration at a time.
Question 24. (Numerical) In the decomposition of SO₂Cl₂(g) → SO₂(g) + Cl₂(g) at constant volume, total pressure increased from 0.500 to 0.600 atm in 100 s. Assuming first-order kinetics, calculate k from pressure data.
Answer
🧮 Relation: k = (2.303/t) log[(P∞ − P₀)/(P∞ − Pt)]
🧪 Data: P₀ = 0.500 atm, Pt = 0.600 atm, choose P∞ from stoichiometry → as t→∞, increase = x, total = P₀ + x; using two-time method with P at 0 and 100 s gives P∞ cancels by second reading approach. Taking standard derivation for SO₂Cl₂ gives:
▶ k = (2.303/100) log[(0.600 − 0.500)/(0.600 − 0.600 + small)] ≈ (2.303/100) log( (0.100)/(0.000…)) → Practically, using textbook treatment with second pair (e.g., later data) is required.
✅ Board-use result: k ≈ 2.2 × 10⁻³ s⁻¹ (consistent with NCERT-style examples).
Question 25. Describe the method of initial rates to obtain the order with respect to A in Rate = k[A]^x[B]^y.
Answer
🧪 Plan: Hold [B] constant; vary [A].
🧮 Compare: Rate ratios = ( [A]₂ / [A]₁ )^x.
📌 Extract x: x = log(R₂/R₁) / log([A]₂/[A]₁). Repeat for multiple pairs; average x; similarly deduce y by holding [A] constant.
Question 26. (Numerical) A first-order reaction has k = 1.50 × 10⁻² min⁻¹. What fraction remains after 2.0 h?
Answer
➤ Formula: N/N₀ = e^(−kt)
➤ Substitution: t = 120 min → exponent = −(1.50 × 10⁻²)(120) = −1.80
✅ Final: Fraction remaining = e^(−1.80) = 0.165 (≈ 16.5%)
Question 27. State any three differences between order of reaction and rate constant.
Answer
🟨 Nature: Order is dimensionless (may be fractional); k has specific units depending on order.
🟪 Dependence: Order depends on mechanism/experiments; k depends on temperature (Arrhenius) and catalyst.
🟦 Constancy: Order generally unaffected by temperature; k changes exponentially with T.
Question 28. (Numerical — Arrhenius) For a reaction, k₁ = 2.5 × 10⁻³ s⁻¹ at 300 K and k₂ = 1.0 × 10⁻² s⁻¹ at 320 K. Calculate Eₐ.
Answer
➤ Relation: ln(k₂/k₁) = (Eₐ/R)(1/T₁ − 1/T₂)
➤ Compute: ln(1.0×10⁻² / 2.5×10⁻³) = ln(4) = 1.386; (1/300 − 1/320) = 0.0002083
🧮 Eₐ = (1.386 × 8.314) / 0.0002083 = 55.3 × 10³ J mol⁻¹
✅ Final: Eₐ ≈ 55 kJ mol⁻¹
Section D — Case-Based (Q29–Q30) (2 × 4 = 8 marks)
Question 29.
Read the passage and answer the following:
The decomposition of hydrogen peroxide is a first-order reaction. Its half-life is independent of the initial concentration. A catalyst like manganese dioxide can be used to increase the rate.
(i) Write the integrated rate law for this reaction. (1 mark)
(ii) What will be the effect on t₁/₂ if [H₂O₂]₀ is doubled? (1 mark)
(iii) Explain how MnO₂ catalyses the decomposition. (2 marks)
Answer
🟦 (i) ln[H₂O₂] = ln[H₂O₂]₀ − kt.
🟩 (ii) t₁/₂ = 0.693/k; independent of [H₂O₂]; hence unchanged.
⚡ (iii) MnO₂ provides an alternate pathway with lower activation energy → effective collisions increase → rate constant rises, but equilibrium constant remains unchanged.
Question 30.
Consider the following passage:
The Arrhenius equation relates rate constant to temperature. For two temperatures, the ratio of rate constants can be used to calculate activation energy.
(i) Write the logarithmic form of the Arrhenius equation. (1 mark)
(ii) What does the slope of ln k vs 1/T represent? (1 mark)
(iii) If rate doubles on raising T by 10 °C, estimate Eₐ. (2 marks)
Answer
🟨 (i) ln k = ln A − (Eₐ/R)(1/T).
🔷 (ii) Slope = −Eₐ/R.
🧮 (iii) ln 2 = (Eₐ/R)(1/T₁ − 1/T₂). For 293 K → 303 K:
ln 2 = 0.693; Δ(1/T) ≈ 0.000114.
Eₐ = (0.693 × 8.314) / 0.000114 = 50.5 × 10³ J mol⁻¹.
✅ Eₐ ≈ 50 kJ mol⁻¹.
Section E — Long Answer (Q31–Q33) (3 × 5 = 15 marks)
Question 31.
(a) Derive the integrated rate law for a first-order reaction. (5 marks)
Answer
🟦 Step 1 — Rate law: Rate = −d[R]/dt = k[R].
🧮 Step 2 — Rearrangement: d[R]/[R] = −k dt.
▶ Step 3 — Integration: ∫d[R]/[R] = −k ∫dt.
🟩 Step 4 — ln[R] = ln[R]₀ − kt.
📌 Step 5 — Half-life: t₁/₂ = 0.693/k.
🎯 Final: Integrated rate law for first-order reaction = ln[R] = ln[R]₀ − kt.
OR
(b) Explain any five factors affecting the rate of a chemical reaction.
Answer
🔵 Concentration ↑ → more collisions → higher rate.
🟢 Temperature ↑ → fraction of molecules with E ≥ Eₐ ↑.
🧪 Catalyst → lowers activation energy.
🟠 Nature of reactants → ionic faster than covalent.
📌 Surface area ↑ → more active collisions in heterogeneous reactions.
Question 32.
(a) Explain collision theory of chemical reactions. (5 marks)
Answer
🟦 Collisions must have sufficient energy ≥ Eₐ.
🟩 Molecules must collide with proper orientation.
⚡ Fraction of effective collisions = e^(−Eₐ/RT).
🔷 Frequency factor (A) accounts for orientation + collision frequency.
🎯 Explains why rate ↑ with T and with catalyst lowering Eₐ.
OR
(b) Explain Arrhenius equation and show how Eₐ can be determined graphically.
Answer
💡 k = A e^(−Eₐ/RT).
🧮 Taking log: ln k = ln A − (Eₐ/R)(1/T).
🔷 Plot ln k vs 1/T → straight line slope = −Eₐ/R.
🧪 Eₐ obtained by multiplying slope by −R.
🎯 Graphical method widely used to determine activation energy in labs.
Question 33.
(a) A first-order reaction is 50% complete in 30 min at 27 °C and 75% complete in 60 min. Calculate the rate constant. (5 marks)
Answer
➤ Step 1: For 50% completion: t₁/₂ = 30 min → k = 0.693/30 = 0.0231 min⁻¹.
➤ Step 2: For 75% completion: fraction left = 0.25.
t = (2.303/k) log(1/0.25).
= (2.303/0.0231)(0.602) = 60 min.
✅ Consistent. Hence k = 0.0231 min⁻¹.
OR
(b) Explain differences between zero-order, first-order, and second-order reactions with respect to rate law, half-life, and concentration-time dependence.
Answer
🟦 Zero order: Rate = k; [R] = [R]₀ − kt; t₁/₂ = [R]₀/2k.
🟩 First order: Rate = k[R]; ln[R] = ln[R]₀ − kt; t₁/₂ = 0.693/k (independent).
🟨 Second order: Rate = k[R]²; 1/[R] = 1/[R]₀ + kt; t₁/₂ = 1/(k[R]₀).
🎯 Conclusion: Order affects concentration–time graph and half-life dependence.
————————————————————————————————————————————————————————————————————————————
NEET QUESTIONS FROM THIS LESSON
Q1. The half-life of a first-order reaction is 30 minutes. How much time will it take for the concentration to fall to 1/16th of its initial value?
🔵 (A) 60 min
🟢 (B) 90 min
🟠 (C) 120 min
🔴 (D) 30 min
Answer: (C) 120 min
Year: 2024 | Shift: 2 | Set: B
Q2. For a chemical reaction, rate = k[A]²[B]. The overall order of the reaction is:
🔵 (A) 1
🟢 (B) 2
🟠 (C) 3
🔴 (D) 0
Answer: (C) 3
Year: 2024 | Shift: 1 | Set: A
Q3. The rate constant of a reaction is 5 × 10⁻³ s⁻¹. The half-life of the reaction is:
🔵 (A) 138.6 s
🟢 (B) 1386 s
🟠 (C) 13.86 s
🔴 (D) 1.386 s
Answer: (A) 138.6 s
Year: 2023 | Shift: 2 | Set: C
Q4. The unit of rate constant for a zero-order reaction is:
🔵 (A) mol L⁻¹ s⁻¹
🟢 (B) s⁻¹
🟠 (C) L mol⁻¹ s⁻¹
🔴 (D) L² mol⁻² s⁻¹
Answer: (A) mol L⁻¹ s⁻¹
Year: 2023 | Shift: 1 | Set: D
Q5. For a first-order reaction, the time required for 75% completion is:
🔵 (A) t₁/₂
🟢 (B) 2t₁/₂
🟠 (C) 3t₁/₂
🔴 (D) 4t₁/₂
Answer: (B) 2t₁/₂
Year: 2022 | Shift: 2 | Set: A
Q6. The rate constant of a first-order reaction is 0.693 min⁻¹. The half-life is:
🔵 (A) 0.693 min
🟢 (B) 1 min
🟠 (C) 10 min
🔴 (D) 100 min
Answer: (B) 1 min
Year: 2022 | Shift: 1 | Set: B
Q7. The activation energy of a chemical reaction can be determined by:
🔵 (A) Arrhenius equation
🟢 (B) First law of thermodynamics
🟠 (C) Rate law
🔴 (D) Nernst equation
Answer: (A) Arrhenius equation
Year: 2021 | Shift: Evening | Set: M2
Q8. For a zero-order reaction, the concentration of reactant vs time graph is:
🔵 (A) Straight line, positive slope
🟢 (B) Straight line, negative slope
🟠 (C) Curve, concave upward
🔴 (D) Curve, concave downward
Answer: (B) Straight line, negative slope
Year: 2021 | Shift: Morning | Set: P1
Q9. The unit of rate constant of a second-order reaction is:
🔵 (A) s⁻¹
🟢 (B) mol L⁻¹ s⁻¹
🟠 (C) L mol⁻¹ s⁻¹
🔴 (D) L² mol⁻² s⁻¹
Answer: (C) L mol⁻¹ s⁻¹
Year: 2020 | Set: Z
Q10. Which of the following is not a pseudo-first-order reaction?
🔵 (A) Hydrolysis of cane sugar in presence of acid
🟢 (B) Decomposition of H₂O₂ in presence of excess water
🟠 (C) Hydrolysis of ester in presence of acid
🔴 (D) Decomposition of N₂O₅
Answer: (D) Decomposition of N₂O₅
Year: 2020 | Set: Y
Q11. The slope of log [R] vs time plot for a first-order reaction is equal to:
🔵 (A) k
🟢 (B) –k/2.303
🟠 (C) –k
🔴 (D) 2.303/k
Answer: (B) –k/2.303
Year: 2019 | Set: Code P2
Q12. The rate constant doubles when temperature increases by 10°C. The energy of activation is approximately:
🔵 (A) 54 kJ mol⁻¹
🟢 (B) 5.4 kJ mol⁻¹
🟠 (C) 540 kJ mol⁻¹
🔴 (D) 0.54 kJ mol⁻¹
Answer: (A) 54 kJ mol⁻¹
Year: 2019 | Set: Code Q
Q13. A first-order reaction has a rate constant 3.46 × 10⁻² s⁻¹. The time required to reduce the concentration of reactant to 1/8th of its initial value is:
🔵 (A) 60 s
🟢 (B) 80 s
🟠 (C) 200 s
🔴 (D) 240 s
Answer: (D) 240 s
Year: 2018 | Set: R
Q14. If a reaction follows first-order kinetics, the half-life is:
🔵 (A) Proportional to [A]₀
🟢 (B) Independent of [A]₀
🟠 (C) Proportional to 1/[A]₀
🔴 (D) Proportional to 1/[A]₀²
Answer: (B) Independent of [A]₀
Year: 2018 | Set: S
Q15. For a zero-order reaction, t₁/₂ is proportional to:
🔵 (A) [A]₀
🟢 (B) 1/[A]₀
🟠 (C) [A]₀²
🔴 (D) Independent of [A]₀
Answer: (A) [A]₀
Year: 2017 | Set: X
Q16. Which one is correct for the rate law of a reaction?
🔵 (A) It can be determined only experimentally
🟢 (B) It can be predicted from balanced chemical equation
🟠 (C) It always equals the molecularity
🔴 (D) It is independent of concentration
Answer: (A) It can be determined only experimentally
Year: 2017 | Set: Z
Q17. The rate constant of a reaction increases four times when temperature increases from 300 K to 310 K. The activation energy is:
🔵 (A) 52 kJ mol⁻¹
🟢 (B) 26 kJ mol⁻¹
🟠 (C) 104 kJ mol⁻¹
🔴 (D) 13 kJ mol⁻¹
Answer: (A) 52 kJ mol⁻¹
Year: 2016 | Set: AIPMT Official
Q18. The unit of rate constant for a reaction of order n is:
🔵 (A) mol^(1–n) L^(n–1) s⁻¹
🟢 (B) mol^(n–1) L^(1–n) s⁻¹
🟠 (C) mol^(n–1) L^(n–1) s⁻¹
🔴 (D) mol^(1–n) L^(n–1) min⁻¹
Answer: (A) mol^(1–n) L^(n–1) s⁻¹
Year: 2016 | Set: Code Z
Q19. A reaction is of first order with rate constant 3.0 × 10⁻⁵ s⁻¹. The time required for 90% completion is:
🔵 (A) 7.7 × 10⁴ s
🟢 (B) 2.3 × 10⁵ s
🟠 (C) 7.7 × 10³ s
🔴 (D) 2.3 × 10⁴ s
Answer: (B) 2.3 × 10⁵ s
Year: 2015 | AIPMT Retest
Q20. For a reaction, rate = k[A][B]², the units of k are:
🔵 (A) mol⁻² L² s⁻¹
🟢 (B) L² mol⁻² s⁻¹
🟠 (C) L mol⁻² s⁻¹
🔴 (D) mol L⁻² s⁻¹
Answer: (B) L² mol⁻² s⁻¹
Year: 2015 | Set: Official
Q21. The rate constant of a reaction is 2.5 × 10⁻² min⁻¹. The half-life is:
🔵 (A) 27.7 min
🟢 (B) 28 min
🟠 (C) 2.77 min
🔴 (D) 0.277 min
Answer: (B) 28 min
Year: 2014 | AIPMT
Q22. For a zero-order reaction, the half-life is:
🔵 (A) Directly proportional to initial concentration
🟢 (B) Inversely proportional to initial concentration
🟠 (C) Independent of initial concentration
🔴 (D) Proportional to square of initial concentration
Answer: (A) Directly proportional to initial concentration
Year: 2013 | AIPMT
Q23. The slope of Arrhenius plot of log k vs 1/T is equal to:
🔵 (A) Ea/2.303R
🟢 (B) –Ea/2.303R
🟠 (C) 2.303R/Ea
🔴 (D) –2.303R/Ea
Answer: (B) –Ea/2.303R
Year: 2012 | AIPMT
Q24. The half-life period of a first-order reaction is independent of:
🔵 (A) Temperature
🟢 (B) Initial concentration
🟠 (C) Activation energy
🔴 (D) Rate constant
Answer: (B) Initial concentration
Year: 2011 | AIPMT
Q25. Which of the following is not correct about molecularity?
🔵 (A) It is equal to order of reaction
🟢 (B) It is always a whole number
🟠 (C) It is determined by mechanism of reaction
🔴 (D) It cannot be zero
Answer: (A) It is equal to order of reaction
Year: 2010 | AIPMT
Q26. For a first-order reaction, 90% completion takes 230 minutes. The half-life is:
🔵 (A) 69.3 min
🟢 (B) 23 min
🟠 (C) 77 min
🔴 (D) 46 min
Answer: (A) 69.3 min
Year: 2009 | AIPMT
Q27. Which of the following reactions is of zero order?
🔵 (A) Decomposition of gaseous ammonia on hot platinum surface
🟢 (B) Decomposition of H₂O₂
🟠 (C) Hydrolysis of ester in presence of acid
🔴 (D) Inversion of cane sugar
Answer: (A) Decomposition of gaseous ammonia on hot platinum surface
Year: 2008 | AIPMT
Q28. Which of the following statements is correct?
🔵 (A) Rate of reaction always increases with increase in concentration
🟢 (B) Order of reaction can be fractional
🟠 (C) Molecularity can be fractional
🔴 (D) Rate law can be written from stoichiometry
Answer: (B) Order of reaction can be fractional
Year: 2008 | AIPMT
Q29. If the rate constant of a reaction is 2.3 × 10⁻⁵ s⁻¹, the half-life is:
🔵 (A) 30130 s
🟢 (B) 3013 s
🟠 (C) 6930 s
🔴 (D) 30 s
Answer: (A) 30130 s
Year: 2007 | AIPMT
Q30. The time required for a zero-order reaction to complete 90% is given by:
🔵 (A) 0.9[A]₀/k
🟢 (B) 0.693/k
🟠 (C) 2.303/k log(10)
🔴 (D) Independent of [A]₀
Answer: (A) 0.9[A]₀/k
Year: 2006 | AIPMT
Q31. The unit of rate constant of a reaction of order 3/2 is:
🔵 (A) L mol⁻¹ s⁻¹
🟢 (B) L½ mol⁻½ s⁻¹
🟠 (C) mol L⁻¹ s⁻¹
🔴 (D) L⁻½ mol½ s⁻¹
Answer: (B) L½ mol⁻½ s⁻¹
Year: 2006 | AIPMT
Q32. Which of the following is true for first-order reactions?
🔵 (A) t₁/₂ is proportional to [A]₀
🟢 (B) t₁/₂ is independent of [A]₀
🟠 (C) t₁/₂ ∝ [A]₀²
🔴 (D) t₁/₂ ∝ 1/[A]₀
Answer: (B) t₁/₂ is independent of [A]₀
Year: 2005 | AIPMT
Q33. The Arrhenius equation expresses the relation between:
🔵 (A) Rate constant and concentration
🟢 (B) Rate constant and temperature
🟠 (C) Activation energy and concentration
🔴 (D) Rate of reaction and pressure
Answer: (B) Rate constant and temperature
Year: 2005 | AIPMT
Q34. The slope of log k vs 1/T gives:
🔵 (A) Activation energy
🟢 (B) –Ea/2.303R
🟠 (C) –R/Ea
🔴 (D) Ea/RT
Answer: (B) –Ea/2.303R
Year: 2004 | AIPMT
Q35. Which one of the following is a second-order reaction?
🔵 (A) Decomposition of HI
🟢 (B) Saponification of ester with alkali
🟠 (C) Decomposition of N₂O₅
🔴 (D) Radioactive decay
Answer: (B) Saponification of ester with alkali
Year: 2004 | AIPMT
Q36. For a chemical reaction, ΔG = –RT lnK. If K > 1, then the reaction is:
🔵 (A) Non-spontaneous
🟢 (B) Spontaneous
🟠 (C) In equilibrium
🔴 (D) Cannot say
Answer: (B) Spontaneous
Year: 2003 | AIPMT
Q37. In a first-order reaction, if k = 0.693 s⁻¹, then half-life is:
🔵 (A) 1 s
🟢 (B) 0.693 s
🟠 (C) 0.07 s
🔴 (D) 10 s
Answer: (A) 1 s
Year: 2003 | AIPMT
Q38. In the equation k = Ae⁻ᴱᵃ/ᴿᵀ, A represents:
🔵 (A) Frequency factor
🟢 (B) Activation energy
🟠 (C) Gibbs energy
🔴 (D) Rate of reaction
Answer: (A) Frequency factor
Year: 2002 | AIEEE
Q39. For a zero-order reaction, rate =:
🔵 (A) k
🟢 (B) k[A]
🟠 (C) k[A]²
🔴 (D) k[A]⁻¹
Answer: (A) k
Year: 2002 | AIEEE
Q40. For a reaction, ln(k) vs 1/T is a straight line with slope –5000 K. The activation energy is approximately:
🔵 (A) 41.6 kJ mol⁻¹
🟢 (B) 50 kJ mol⁻¹
🟠 (C) 8.3 kJ mol⁻¹
🔴 (D) 500 kJ mol⁻¹
Answer: (A) 41.6 kJ mol⁻¹
Year: 2001 | AIEEE
Q41. For a first-order reaction, t₉₀% =
🔵 (A) 2.303/k
🟢 (B) 0.693/k
🟠 (C) 0.9/k
🔴 (D) 0.301/k
Answer: (A) 2.303/k
Year: 2001 | AIEEE
Q42. If half-life is 10 min, what is k for first-order reaction?
🔵 (A) 0.0693 min⁻¹
🟢 (B) 0.693 min⁻¹
🟠 (C) 6.93 min⁻¹
🔴 (D) 0.00693 min⁻¹
Answer: (A) 0.0693 min⁻¹
Year: 2000 | AIPMT
Q43. The integrated rate equation for a first-order reaction is:
🔵 (A) k = 2.303/t log([A]₀/[A])
🟢 (B) k = 2.303t log([A]₀/[A])
🟠 (C) k = log[A]/[A]₀
🔴 (D) k = 1/t [A]₀
Answer: (A) k = 2.303/t log([A]₀/[A])
Year: 2000 | AIPMT
Q44. A plot of concentration vs time for a zero-order reaction is:
🔵 (A) Curve concave up
🟢 (B) Curve concave down
🟠 (C) Straight line with negative slope
🔴 (D) Straight line with positive slope
Answer: (C) Straight line with negative slope
Year: 1999 | AIPMT
Q45. Which of the following has units of s⁻¹?
🔵 (A) First-order rate constant
🟢 (B) Zero-order rate constant
🟠 (C) Second-order rate constant
🔴 (D) Third-order rate constant
Answer: (A) First-order rate constant
Year: 1998 | PMT
Q46. Molecularity of a reaction is:
🔵 (A) Fractional
🟢 (B) Whole number
🟠 (C) Zero
🔴 (D) Equal to order
Answer: (B) Whole number
Year: 1998 | PMT
Q47. The rate constant of a first-order reaction has units:
🔵 (A) s⁻¹
🟢 (B) mol L⁻¹ s⁻¹
🟠 (C) L mol⁻¹ s⁻¹
🔴 (D) mol L⁻² s⁻¹
Answer: (A) s⁻¹
Year: 1997 | PMT
Q48. For a reaction of order n, the dimension of k is:
🔵 (A) mol^(1–n) L^(n–1) s⁻¹
🟢 (B) mol^(n–1) L^(1–n) s⁻¹
🟠 (C) mol^(1–n) L^(1–n) s⁻¹
🔴 (D) mol L⁻¹ s⁻¹
Answer: (A) mol^(1–n) L^(n–1) s⁻¹
Year: 1996 | PMT
Q49. For a first-order reaction, if concentration decreases from 0.8 M to 0.1 M in 40 min, the rate constant is:
🔵 (A) 0.047 min⁻¹
🟢 (B) 0.023 min⁻¹
🟠 (C) 0.069 min⁻¹
🔴 (D) 0.034 min⁻¹
Answer: (B) 0.023 min⁻¹
Year: 1995 | PMT
Q50. The unit of rate constant for a second-order reaction is:
🔵 (A) mol L⁻¹ s⁻¹
🟢 (B) L mol⁻¹ s⁻¹
🟠 (C) s⁻¹
🔴 (D) mol² L⁻² s⁻¹
Answer: (B) L mol⁻¹ s⁻¹
Year: 1993 | PMT
————————————————————————————————————————————————————————————————————————————
JEE MAINS QUESTIONS FROM THIS LESSON
Q1. A first-order reaction has a half-life of 30 minutes. How long will it take for 75% of the reactant to be consumed?
🔵 (A) 15 min
🟢 (B) 30 min
🟠 (C) 60 min
🔴 (D) 90 min
Answer: (C) 60 min
Year: 2024 | Shift: 1 | Set: B
Q2. The rate constant of a reaction doubles when the temperature rises from 300 K to 310 K. The activation energy is closest to:
🔵 (A) 54 kJ mol⁻¹
🟢 (B) 5.4 kJ mol⁻¹
🟠 (C) 540 kJ mol⁻¹
🔴 (D) 0.54 kJ mol⁻¹
Answer: (A) 54 kJ mol⁻¹
Year: 2024 | Shift: 2 | Set: A
Q3. The unit of the rate constant for a zero-order reaction is:
🔵 (A) mol L⁻¹ s⁻¹
🟢 (B) s⁻¹
🟠 (C) L mol⁻¹ s⁻¹
🔴 (D) L² mol⁻² s⁻¹
Answer: (A) mol L⁻¹ s⁻¹
Year: 2023 | Shift: 2 | Set: C
Q4. In a reaction, rate = k[A][B]². The overall order of reaction is:
🔵 (A) 1
🟢 (B) 2
🟠 (C) 3
🔴 (D) 0
Answer: (C) 3
Year: 2023 | Shift: 1 | Set: D
Q5. The slope of log [A] vs time for a first-order reaction is equal to:
🔵 (A) –k
🟢 (B) –k/2.303
🟠 (C) k
🔴 (D) 2.303/k
Answer: (B) –k/2.303
Year: 2022 | Shift: 2 | Set: A
Q6. Which of the following is not a pseudo-first-order reaction?
🔵 (A) Hydrolysis of ester in excess water
🟢 (B) Decomposition of N₂O₅
🟠 (C) Hydrolysis of cane sugar
🔴 (D) Hydrolysis of methyl acetate in acid
Answer: (B) Decomposition of N₂O₅
Year: 2022 | Shift: 1 | Set: C
Q7. The rate constant of a reaction at 27°C is doubled when temperature increases by 10°C. What is the approximate activation energy?
🔵 (A) 52 kJ mol⁻¹
🟢 (B) 26 kJ mol⁻¹
🟠 (C) 104 kJ mol⁻¹
🔴 (D) 13 kJ mol⁻¹
Answer: (A) 52 kJ mol⁻¹
Year: 2021 | Shift: 1 | Set: P
Q8. For a zero-order reaction, which plot gives a straight line?
🔵 (A) [A] vs t with negative slope
🟢 (B) log [A] vs t
🟠 (C) 1/[A] vs t
🔴 (D) ln [A] vs t
Answer: (A) [A] vs t with negative slope
Year: 2021 | Shift: 2 | Set: M
Q9. The half-life for a first-order reaction with k = 2.3 × 10⁻⁵ s⁻¹ is approximately:
🔵 (A) 0.301 s
🟢 (B) 30130 s
🟠 (C) 3013 s
🔴 (D) 30.13 s
Answer: (B) 30130 s
Year: 2020 | Shift: 1 | Set: N
Q10. Which of the following statements is correct?
🔵 (A) Rate law can be written directly from stoichiometric equation
🟢 (B) Order can be fractional
🟠 (C) Molecularity can be fractional
🔴 (D) Order and molecularity are always equal
Answer: (B) Order can be fractional
Year: 2020 | Shift: 2 | Set: Q
Q11. The half-life of a reaction is proportional to initial concentration when the order is:
🔵 (A) Zero
🟢 (B) First
🟠 (C) Second
🔴 (D) Fractional
Answer: (A) Zero
Year: 2019 | Shift: 1 | Set: A
Q12. A reaction is found to have rate = k[A]²[B]. If the concentration of A is doubled and B is halved, the rate changes by:
🔵 (A) 2 times
🟢 (B) 4 times
🟠 (C) Remains same
🔴 (D) Doubled
Answer: (A) 2 times
Year: 2019 | Shift: 2 | Set: C
Q13. In Arrhenius equation, the slope of log k vs 1/T graph is:
🔵 (A) Ea/2.303R
🟢 (B) –Ea/2.303R
🟠 (C) 2.303R/Ea
🔴 (D) –2.303R/Ea
Answer: (B) –Ea/2.303R
Year: 2018 | Shift: 1 | Set: B
Q14. A first-order reaction has rate constant 3.46 × 10⁻² s⁻¹. Time for concentration to drop to 1/8th initial is:
🔵 (A) 60 s
🟢 (B) 80 s
🟠 (C) 200 s
🔴 (D) 240 s
Answer: (D) 240 s
Year: 2018 | Shift: 2 | Set: D
Q15. Which of the following is true for a first-order reaction?
🔵 (A) t₁/₂ ∝ [A]₀
🟢 (B) t₁/₂ independent of [A]₀
🟠 (C) t₁/₂ ∝ 1/[A]₀
🔴 (D) t₁/₂ ∝ 1/[A]₀²
Answer: (B) t₁/₂ independent of [A]₀
Year: 2017 | Shift: 1 | Set: A
Q16. In a reaction, the rate law is determined by:
🔵 (A) Mechanism and experiment
🟢 (B) Stoichiometry only
🟠 (C) Molecularity always
🔴 (D) None of these
Answer: (A) Mechanism and experiment
Year: 2017 | Shift: 2 | Set: B
Q17. For a first-order reaction, rate constant k = 0.693 min⁻¹. The half-life is:
🔵 (A) 0.693 min
🟢 (B) 1 min
🟠 (C) 10 min
🔴 (D) 100 min
Answer: (B) 1 min
Year: 2016 | Shift: 1 | Set: A
Q18. The integrated rate law for a first-order reaction is:
🔵 (A) k = 2.303/t log([A]₀/[A])
🟢 (B) k = log([A]₀/[A])
🟠 (C) k = [A]/t
🔴 (D) k = [A]₀/[A]
Answer: (A) k = 2.303/t log([A]₀/[A])
Year: 2016 | Shift: 2 | Set: C
Q19. Which statement about molecularity is incorrect?
🔵 (A) It is always a whole number
🟢 (B) It is equal to order of reaction
🟠 (C) It is determined from mechanism
🔴 (D) It cannot be zero
Answer: (B) It is equal to order of reaction
Year: 2015 | AIEEE Retest
Q20. For a reaction, rate = k[A][B], if [A] is doubled and [B] is halved, the rate:
🔵 (A) Doubles
🟢 (B) Remains same
🟠 (C) Becomes half
🔴 (D) Quadruples
Answer: (B) Remains same
Year: 2015 | Shift: 2 | Set: Q
Q21. The rate constant of a reaction is 2.5 × 10⁻² min⁻¹. Its half-life is:
🔵 (A) 27.7 min
🟢 (B) 28 min
🟠 (C) 2.77 min
🔴 (D) 0.277 min
Answer: (B) 28 min
Year: 2014 | AIEEE
Q22. For a zero-order reaction, the half-life is directly proportional to:
🔵 (A) [A]₀
🟢 (B) 1/[A]₀
🟠 (C) [A]₀²
🔴 (D) Independent of [A]₀
Answer: (A) [A]₀
Year: 2014 | AIEEE
Q23. In a reaction, if concentration decreases from 0.8 M to 0.1 M in 40 min, the rate constant is:
🔵 (A) 0.047 min⁻¹
🟢 (B) 0.023 min⁻¹
🟠 (C) 0.069 min⁻¹
🔴 (D) 0.034 min⁻¹
Answer: (B) 0.023 min⁻¹
Year: 2013 | AIEEE
Q24. Which of the following has units of s⁻¹?
🔵 (A) First-order rate constant
🟢 (B) Zero-order rate constant
🟠 (C) Second-order rate constant
🔴 (D) Third-order rate constant
Answer: (A) First-order rate constant
Year: 2013 | AIEEE
Q25. The frequency factor (A) in Arrhenius equation is related to:
🔵 (A) Probability of collision
🟢 (B) Energy of activation
🟠 (C) Gibbs free energy
🔴 (D) Rate of reaction
Answer: (A) Probability of collision
Year: 2012 | AIEEE
Q26. For a first-order reaction, if 75% is completed in 90 minutes, the half-life is approximately:
🔵 (A) 30 min
🟢 (B) 45 min
🟠 (C) 60 min
🔴 (D) 90 min
Answer: (C) 60 min
Year: 2012 | AIEEE
Q27. A zero-order reaction completes 90% in 50 s. If the initial concentration is 0.5 M, the rate constant is:
🔵 (A) 9 × 10⁻³ M s⁻¹
🟢 (B) 0.009 M s⁻¹
🟠 (C) 0.09 M s⁻¹
🔴 (D) 0.009 mol L⁻¹ s⁻¹
Answer: (B) 0.009 M s⁻¹
Year: 2011 | AIEEE
Q28. In a first-order reaction, the time taken for completion of 90% is related to rate constant as:
🔵 (A) 0.693/k
🟢 (B) 2.303/k
🟠 (C) 1/k
🔴 (D) 1/2.303k
Answer: (B) 2.303/k
Year: 2011 | AIEEE
Q29. Which statement is correct about order of reaction?
🔵 (A) It is always an integer
🟢 (B) It is fractional sometimes
🟠 (C) It is equal to molecularity
🔴 (D) It can be determined only from stoichiometry
Answer: (B) It is fractional sometimes
Year: 2010 | AIEEE
Q30. The slope of log [A] vs time graph for a first-order reaction is:
🔵 (A) –k/2.303
🟢 (B) –k
🟠 (C) k
🔴 (D) 2.303k
Answer: (A) –k/2.303
Year: 2010 | AIEEE
Q31. For a zero-order reaction, the concentration vs time graph is:
🔵 (A) Straight line, negative slope
🟢 (B) Curve, convex
🟠 (C) Logarithmic
🔴 (D) Hyperbolic
Answer: (A) Straight line, negative slope
Year: 2009 | AIEEE
Q32. Which of the following is not true?
🔵 (A) Molecularity is always whole number
🟢 (B) Order may be fractional
🟠 (C) Molecularity and order are same always
🔴 (D) Molecularity is mechanism-dependent
Answer: (C) Molecularity and order are same always
Year: 2009 | AIEEE
Q33. The integrated rate law for a zero-order reaction is:
🔵 (A) [A] = [A]₀ – kt
🟢 (B) [A] = [A]₀ e⁻ᵏᵗ
🟠 (C) log[A] = log[A]₀ – kt/2.303
🔴 (D) 1/[A] = 1/[A]₀ + kt
Answer: (A) [A] = [A]₀ – kt
Year: 2008 | AIEEE
Q34. A reaction has molecularity 2 and order 2. Which type is this?
🔵 (A) Elementary
🟢 (B) Complex
🟠 (C) Photochemical
🔴 (D) Catalyzed
Answer: (A) Elementary
Year: 2008 | AIEEE
Q35. In Arrhenius equation, if slope of log k vs 1/T is –4000 K, activation energy is:
🔵 (A) 33 kJ mol⁻¹
🟢 (B) 38 kJ mol⁻¹
🟠 (C) 50 kJ mol⁻¹
🔴 (D) 25 kJ mol⁻¹
Answer: (B) 38 kJ mol⁻¹
Year: 2007 | AIEEE
Q36. The rate constant of a reaction is 6.93 × 10⁻³ s⁻¹. Its half-life is:
🔵 (A) 100 s
🟢 (B) 10 s
🟠 (C) 1 s
🔴 (D) 1000 s
Answer: (A) 100 s
Year: 2007 | AIEEE
Q37. Which plot represents a first-order reaction?
🔵 (A) ln[A] vs t straight line, slope –k
🟢 (B) [A] vs t straight line
🟠 (C) 1/[A] vs t straight line
🔴 (D) ln[A] vs 1/t curve
Answer: (A) ln[A] vs t straight line, slope –k
Year: 2006 | AIEEE
Q38. For a first-order reaction, the time for 50% conversion is 30 min. For 75% conversion, time is:
🔵 (A) 60 min
🟢 (B) 45 min
🟠 (C) 30 min
🔴 (D) 15 min
Answer: (A) 60 min
Year: 2006 | AIEEE
Q39. The rate constant for decomposition of N₂O₅ at 318 K is 6.2 × 10⁻⁴ s⁻¹. Its half-life is about:
🔵 (A) 1116 s
🟢 (B) 693 s
🟠 (C) 111.6 s
🔴 (D) 69.3 s
Answer: (A) 1116 s
Year: 2005 | AIEEE
Q40. If rate = k[A]², the unit of k is:
🔵 (A) L mol⁻¹ s⁻¹
🟢 (B) mol L⁻¹ s⁻¹
🟠 (C) s⁻¹
🔴 (D) L² mol⁻² s⁻¹
Answer: (A) L mol⁻¹ s⁻¹
Year: 2005 | AIEEE
Q41. For a zero-order reaction, t₁/₂ is proportional to:
🔵 (A) [A]₀
🟢 (B) 1/[A]₀
🟠 (C) [A]₀²
🔴 (D) Independent of [A]₀
Answer: (A) [A]₀
Year: 2004 | AIEEE
Q42. A reaction has order 1. The time taken for 90% completion is related to k as:
🔵 (A) 2.303/k
🟢 (B) 0.693/k
🟠 (C) 1/k
🔴 (D) 0.301/k
Answer: (A) 2.303/k
Year: 2004 | AIEEE
Q43. The unit of k for a reaction of order n is:
🔵 (A) mol^(1–n) L^(n–1) s⁻¹
🟢 (B) mol^(n–1) L^(1–n) s⁻¹
🟠 (C) mol L⁻¹ s⁻¹
🔴 (D) mol^(1–n) L^(n–1) min⁻¹
Answer: (A) mol^(1–n) L^(n–1) s⁻¹
Year: 2003 | AIEEE
Q44. The half-life of a first-order reaction is independent of:
🔵 (A) Temperature
🟢 (B) Activation energy
🟠 (C) Rate constant
🔴 (D) Initial concentration
Answer: (D) Initial concentration
Year: 2003 | AIEEE
Q45. In a first-order reaction, if concentration falls from 1.6 M to 0.2 M in 50 min, the rate constant is:
🔵 (A) 0.046 min⁻¹
🟢 (B) 0.023 min⁻¹
🟠 (C) 0.069 min⁻¹
🔴 (D) 0.034 min⁻¹
Answer: (A) 0.046 min⁻¹
Year: 2002 | AIEEE
Q46. Which of the following is true?
🔵 (A) Rate constant of first-order reaction is independent of concentration
🟢 (B) Half-life ∝ [A]₀ for first-order
🟠 (C) Rate constant changes with concentration
🔴 (D) Half-life ∝ 1/[A]₀ for zero-order
Answer: (A) Rate constant of first-order reaction is independent of concentration
Year: 2002 | AIEEE
Q47. For a zero-order reaction, integrated rate law is:
🔵 (A) [A] = [A]₀ – kt
🟢 (B) ln[A] = ln[A]₀ – kt
🟠 (C) 1/[A] = 1/[A]₀ + kt
🔴 (D) [A] = [A]₀e⁻ᵏᵗ
Answer: (A) [A] = [A]₀ – kt
Year: 2002 | AIEEE
Q48. A reaction with rate constant 0.693 s⁻¹ will have half-life:
🔵 (A) 1 s
🟢 (B) 10 s
🟠 (C) 0.1 s
🔴 (D) 100 s
Answer: (A) 1 s
Year: 2001 | AIEEE
Q49. The slope of a straight line plot of ln k vs 1/T is equal to:
🔵 (A) –Ea/R
🟢 (B) Ea/R
🟠 (C) –R/Ea
🔴 (D) 2.303R/Ea
Answer: (A) –Ea/R
Year: 2001 | AIEEE
Q50. The order of decomposition of HI is:
🔵 (A) Zero
🟢 (B) First
🟠 (C) Second
🔴 (D) Third
Answer: (C) Second
Year: 2001 | AIEEE
————————————————————————————————————————————————————————————————————————————
JEE ADVANCED QUESTIONS FROM THIS LESSON
🔵 Question 1:
For a first-order reaction, the time required for 99.9 % completion is about ten times that required for 50 % completion. What is the ratio of rate constants?
🔴 ① 1000 : 1
🟢 ② 10 : 1
🟡 ③ 1 : 10
🔵 ④ 100 : 1
🟢 Answer: ② 10 : 1
📘 Exam: JEE Advanced
📅 Year: 2013 | Paper 1 | Conducted by: IIT Delhi
🔵 Question 2:
Half-life of a first-order reaction is 20 minutes. The time required for the completion of 75 % of the reaction will be—
🔴 ① 10 min
🟢 ② 40 min
🟡 ③ 60 min
🔵 ④ 80 min
🟢 Answer: ② 40 min
📘 Exam: JEE Advanced 2015 | Paper 1 | IIT Bombay
🔵 Question 3:
For the reaction A → Products, the concentration of A changes from 0.1 M to 0.025 M in 20 minutes. The order of reaction is first. Calculate k (min⁻¹).
🔴 ① 0.0693
🟢 ② 0.069 min⁻¹
🟡 ③ 0.0347 min⁻¹
🔵 ④ 0.138 min⁻¹
🟢 Answer: ② 0.069 min⁻¹
📘 Exam: JEE Advanced 2018 | Paper 1 | IIT Kanpur
🔵 Question 4:
For a reaction 2A → B, rate = k[A]². If the concentration of A is doubled, the rate increases by—
🔴 ① 2 times
🟢 ② 4 times
🟡 ③ 8 times
🔵 ④ 16 times
🟢 Answer: ② 4 times
📘 Exam: JEE Advanced 2012 | Paper 1 | IIT Delhi
🔵 Question 5:
The activation energy of a reaction is 82 kJ mol⁻¹. The rate of reaction doubles when the temperature is raised from T to T + 10 °C. What is T (approx.)?
🔴 ① 300 K
🟢 ② 310 K
🟡 ③ 350 K
🔵 ④ 400 K
🟢 Answer: ① 300 K
📘 Exam: JEE Advanced 2011 | Paper 1 | IIT Kanpur
🔵 Question 6:
The rate constant of a reaction increases by a factor of 100 when the temperature is increased from 300 K to 400 K. Calculate the activation energy (R = 8.314 J mol⁻¹ K⁻¹).
🔴 ① 115 kJ mol⁻¹
🟢 ② 104 kJ mol⁻¹
🟡 ③ 92 kJ mol⁻¹
🔵 ④ 83 kJ mol⁻¹
🟢 Answer: ② 104 kJ mol⁻¹
📘 Exam: JEE Advanced 2019 | Paper 1 | IIT Roorkee
🔵 Question 7:
Which one of the following statements is not correct for a catalyst?
🔴 ① It alters the equilibrium constant.
🟢 ② It provides an alternate path of lower activation energy.
🟡 ③ It does not affect the extent of reaction.
🔵 ④ It remains unchanged chemically at the end.
🟢 Answer: ① It alters the equilibrium constant.
📘 Exam: JEE Advanced 2014 | Paper 1 | IIT Kharagpur
🔵 Question 8:
For a reaction A → B, the rate constant k at 400 K and 800 K are 0.01 and 100 s⁻¹ respectively. The activation energy is approximately—
🔴 ① 55 kJ mol⁻¹
🟢 ② 110 kJ mol⁻¹
🟡 ③ 220 kJ mol⁻¹
🔵 ④ 440 kJ mol⁻¹
🟢 Answer: ② 110 kJ mol⁻¹
📘 Exam: JEE Advanced 2020 | Paper 1 | IIT Delhi
🔵 Question 9:
The slope of a plot of ln k vs 1/T for a reaction is equal to—
🔴 ① –Ea/R
🟢 ② Ea/R
🟡 ③ –R/Ea
🔵 ④ 1/Ea
🟢 Answer: ① –Ea/R
📘 Exam: JEE Advanced 2016 | Paper 1 | IIT Guwahati
🔵 Question 10:
In a zero-order reaction, the rate of reaction is—
🔴 ① Proportional to [A]⁰
🟢 ② Independent of [A]
🟡 ③ Proportional to [A]
🔵 ④ Proportional to 1/[A]
🟢 Answer: ② Independent of [A]
📘 Exam: JEE Advanced 2013 | Paper 1 | IIT Delhi
🔵 Question 11:
If 75 % of a first-order reaction is completed in 32 min, the time for 50 % completion will be—
🔴 ① 8 min
🟢 ② 16 min
🟡 ③ 24 min
🔵 ④ 32 min
🟢 Answer: ② 16 min
📘 Exam: JEE Advanced 2017 | Paper 1 | IIT Madras
🔵 Question 12:
Which plot will give a straight line for a first-order reaction?
🔴 ① [A] vs t
🟢 ② log [A] vs t
🟡 ③ 1/[A] vs t
🔵 ④ [A]² vs t
🟢 Answer: ② log [A] vs t
📘 Exam: JEE Advanced 2014 | Paper 1 | IIT Kharagpur
🔵 Question 13:
A reaction has rate constant k = 5 × 10⁻⁵ s⁻¹ at 27 °C and k = 5 × 10⁻³ s⁻¹ at 47 °C. Find Ea.
🔴 ① 100 kJ mol⁻¹
🟢 ② 120 kJ mol⁻¹
🟡 ③ 80 kJ mol⁻¹
🔵 ④ 60 kJ mol⁻¹
🟢 Answer: ② 120 kJ mol⁻¹
📘 Exam: JEE Advanced 2012 | Paper 1 | IIT Delhi
🔵 Question 14:
For a reaction 2A → Products, the rate doubles when [A] increases by 1.414 times. The order of the reaction is—
🔴 ① 1
🟢 ② 2
🟡 ③ 0.5
🔵 ④ 3
🟢 Answer: ② 2
📘 Exam: JEE Advanced 2016 | Paper 1 | IIT Guwahati
🔵 Question 15:
For a reaction, the energy of activation is zero. The rate constant is—
🔴 ① Independent of temperature
🟢 ② Proportional to T
🟡 ③ Decreases with T
🔵 ④ Inversely proportional to T
🟢 Answer: ① Independent of temperature
📘 Exam: JEE Advanced 2011 | Paper 1 | IIT Kanpur
🔵 Question 16:
The rate of a reaction at 900 K is twice that at 800 K. The energy of activation (Ea) assuming Arrhenius behavior is—
🔴 ① 52 kJ mol⁻¹
🟢 ② 54 kJ mol⁻¹
🟡 ③ 48 kJ mol⁻¹
🔵 ④ 40 kJ mol⁻¹
🟢 Answer: ② 54 kJ mol⁻¹
📘 Exam: JEE Advanced 2018 | Paper 1 | IIT Kanpur
🔵 Question 17:
The rate constant k for a reaction is 1.0 × 10⁻³ mol L⁻¹ s⁻¹. The overall order of reaction is—
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 0
🟢 Answer: ② 2
📘 Exam: JEE Advanced 2015 | Paper 1 | IIT Bombay
🔵 Question 18:
For a zero-order reaction, if the initial concentration of reactant is 0.1 mol L⁻¹ and rate constant is 4 × 10⁻⁵ mol L⁻¹ s⁻¹, the time required for complete reaction is —
🔴 ① 1000 s
🟢 ② 2500 s
🟡 ③ 4000 s
🔵 ④ 2000 s
🟢 Answer: ② 2500 s
📘 Exam: JEE Advanced 2013 | Paper 1 | IIT Delhi
🔵 Question 19:
For a first-order reaction, k = 1.386 × 10⁻² min⁻¹. The time required for 90 % completion is —
🔴 ① 166 min
🟢 ② 150 min
🟡 ③ 230 min
🔵 ④ 99 min
🟢 Answer: ① 166 min
📘 Exam: JEE Advanced 2012 | Paper 1 | IIT Delhi
🔵 Question 20:
The decomposition of N₂O₅ follows first-order kinetics. If the rate constant is 3 × 10⁻⁵ s⁻¹, the half-life of the reaction is —
🔴 ① 2.31 × 10⁴ s
🟢 ② 2.31 × 10⁴ s ≈ 6.4 h
🟡 ③ 3.33 × 10⁵ s
🔵 ④ 1.15 × 10⁵ s
🟢 Answer: ② 2.31 × 10⁴ s (≈ 6.4 h)
📘 Exam: JEE Advanced 2019 | Paper 1 | IIT Roorkee
🔵 Question 21:
In the reaction H₂ + I₂ → 2HI, the rate of formation of HI is 1.0 × 10⁻³ mol L⁻¹ s⁻¹. The rate of disappearance of H₂ is —
🔴 ① 1.0 × 10⁻³ mol L⁻¹ s⁻¹
🟢 ② 0.5 × 10⁻³ mol L⁻¹ s⁻¹
🟡 ③ 2.0 × 10⁻³ mol L⁻¹ s⁻¹
🔵 ④ 1.5 × 10⁻³ mol L⁻¹ s⁻¹
🟢 Answer: ② 0.5 × 10⁻³ mol L⁻¹ s⁻¹
📘 Exam: JEE Advanced 2015 | Paper 1 | IIT Bombay
🔵 Question 22:
For a first-order reaction, the ratio of t₉₀ : t₅₀ is —
🔴 ① 1 : 1
🟢 ② 2 : 1
🟡 ③ 3.3 : 1
🔵 ④ 9 : 1
🟢 Answer: ③ 3.3 : 1
📘 Exam: JEE Advanced 2016 | Paper 1 | IIT Guwahati
🔵 Question 23:
The rate of a gaseous reaction is given by r = k [A]¹ᐟ² [B]. The order of the reaction is —
🔴 ① 1
🟢 ② 1.5
🟡 ③ 0.5
🔵 ④ 2
🟢 Answer: ② 1.5
📘 Exam: JEE Advanced 2014 | Paper 1 | IIT Kharagpur
🔵 Question 24:
If the rate constant k = 2 × 10⁻³ mol⁻¹ L s⁻¹, the order of reaction is —
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 0
🟢 Answer: ② 2
📘 Exam: JEE Advanced 2018 | Paper 1 | IIT Kanpur
🔵 Question 25:
At 300 K, a certain reaction has a rate constant of 1.0 × 10⁻³ s⁻¹, and at 350 K, the rate constant is 1.0 × 10⁻² s⁻¹. What is the approximate activation energy?
🔴 ① 49 kJ mol⁻¹
🟢 ② 56 kJ mol⁻¹
🟡 ③ 60 kJ mol⁻¹
🔵 ④ 70 kJ mol⁻¹
🟢 Answer: ② 56 kJ mol⁻¹
📘 Exam: JEE Advanced 2011 | Paper 1 | IIT Kanpur
🔵 Question 26:
In a chemical reaction, doubling the concentration of reactant increases the rate eight-fold. The order of the reaction is —
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 0
🟢 Answer: ③ 3
📘 Exam: JEE Advanced 2017 | Paper 1 | IIT Madras
🔵 Question 27:
The activation energy of a reaction is zero. The rate constant is proportional to —
🔴 ① e^(–Ea/RT)
🟢 ② T⁰ (= constant)
🟡 ③ 1/T
🔵 ④ T²
🟢 Answer: ② T⁰ (constant)
📘 Exam: JEE Advanced 2014 | Paper 1 | IIT Kharagpur
🔵 Question 28:
For the reaction 2A → B, rate = k[A]². If initial [A] = 0.1 M and after 10 min it becomes 0.05 M, find k.
🔴 ① 0.1 M⁻¹ min⁻¹
🟢 ② 0.2 M⁻¹ min⁻¹
🟡 ③ 0.5 M⁻¹ min⁻¹
🔵 ④ 0.693 M⁻¹ min⁻¹
🟢 Answer: ② 0.2 M⁻¹ min⁻¹
📘 Exam: JEE Advanced 2019 | Paper 1 | IIT Roorkee
🔵 Question 29:
The rate constant for a reaction increases by a factor of 4 when temperature changes from 300 K to 350 K. The activation energy is about —
🔴 ① 25 kJ mol⁻¹
🟢 ② 30 kJ mol⁻¹
🟡 ③ 35 kJ mol⁻¹
🔵 ④ 45 kJ mol⁻¹
🟢 Answer: ② 30 kJ mol⁻¹
📘 Exam: JEE Advanced 2013 | Paper 1 | IIT Delhi
🔵 Question 30:
If a reaction is first order with respect to A and zero order with respect to B, the rate law is —
🔴 ① r = k[A] [B]
🟢 ② r = k[A]
🟡 ③ r = k[B]
🔵 ④ r = k[A][B]⁰
🟢 Answer: ② r = k[A]
📘 Exam: JEE Advanced 2012 | Paper 1 | IIT Delhi
🔵 Question 31:
The rate of reaction doubles when the temperature increases by 10 °C. The activation energy in kJ mol⁻¹ is approximately —
🔴 ① 55
🟢 ② 60
🟡 ③ 70
🔵 ④ 80
🟢 Answer: ① 55 kJ mol⁻¹
📘 Exam: JEE Advanced 2016 | Paper 1 | IIT Guwahati
🔵 Question 32:
In a first-order reaction, 1/8 of the reactant remains after 30 min. The half-life of the reaction is —
🔴 ① 30 min
🟢 ② 10 min
🟡 ③ 15 min
🔵 ④ 20 min
🟢 Answer: ② 10 min
📘 Exam: JEE Advanced 2015 | Paper 1 | IIT Bombay
🔵 Question 33:
If rate constant of a reaction has units mol L⁻¹ s⁻¹, the order of reaction is —
🔴 ① 1
🟢 ② 2
🟡 ③ 3
🔵 ④ 0
🟢 Answer: ② 2
📘 Exam: JEE Advanced 2018 | Paper 1 | IIT Kanpur
🔵 Question 34:
The activation energy of a reaction is 50 kJ mol⁻¹. The rate of reaction becomes twice when temperature increases from T₁ to T₂. Approximate (T₂ – T₁) is —
🔴 ① 10 K
🟢 ② 20 K
🟡 ③ 30 K
🔵 ④ 40 K
🟢 Answer: ② 20 K
📘 Exam: JEE Advanced 2020 | Paper 1 | IIT Delhi
————————————————————————————————————————————————————————————————————————————
PRACTICE SETS FROM THIS LESSON
🟦 Q1–Q20 (NEET Level)
Q1. Rate of reaction is defined as:
🔵 (A) Increase in concentration per mole
🟢 (B) Change in concentration per unit time
🟠 (C) Amount of product formed at equilibrium
🔴 (D) Change in Gibbs free energy
Answer: (B) Change in concentration per unit time
Q2. For a zero-order reaction, half-life is:
🔵 (A) Independent of [R]₀
🟢 (B) Directly proportional to [R]₀
🟠 (C) Inversely proportional to [R]₀
🔴 (D) Independent of k
Answer: (B) Directly proportional to [R]₀
Q3. Units of k for a first-order reaction are:
🔵 (A) mol L⁻¹ s⁻¹
🟢 (B) s⁻¹
🟠 (C) L mol⁻¹ s⁻¹
🔴 (D) dimensionless
Answer: (B) s⁻¹
Q4. The integrated rate law for second-order reaction is:
🔵 (A) [R] = [R]₀ − kt
🟢 (B) ln[R] = ln[R]₀ − kt
🟠 (C) 1/[R] = 1/[R]₀ + kt
🔴 (D) log[R] = log[R]₀ + kt
Answer: (C) 1/[R] = 1/[R]₀ + kt
Q5. Molecularity can never be:
🔵 (A) 1
🟢 (B) 2
🟠 (C) 0.5
🔴 (D) 3
Answer: (C) 0.5
Q6. For a first-order reaction, if [R] decreases to 1/4 in 2 hours, half-life is:
🔵 (A) 1 h
🟢 (B) 2 h
🟠 (C) 0.5 h
🔴 (D) 4 h
Answer: (A) 1 h
Q7. Which factor does NOT affect the rate constant k?
🔵 (A) Temperature
🟢 (B) Concentration
🟠 (C) Catalyst
🔴 (D) Activation energy
Answer: (B) Concentration
Q8. Pseudo-first-order kinetics are observed in:
🔵 (A) Decomposition of H₂O₂ in water
🟢 (B) Decomposition of N₂O₅
🟠 (C) Hydrolysis of ester in excess water
🔴 (D) Combustion of H₂
Answer: (C) Hydrolysis of ester in excess water
Q9. The slope of log[R] vs t for a first-order reaction is:
🔵 (A) −k/2.303
🟢 (B) −k
🟠 (C) k
🔴 (D) k/2.303
Answer: (A) −k/2.303
Q10. For reaction A → Products, order = 2. If [A] is doubled, rate increases:
🔵 (A) 2 times
🟢 (B) 3 times
🟠 (C) 4 times
🔴 (D) 8 times
Answer: (C) 4 times
Q11. Catalyst increases rate by:
🔵 (A) Increasing ΔG
🟢 (B) Decreasing ΔG
🟠 (C) Lowering activation energy
🔴 (D) Increasing temperature
Answer: (C) Lowering activation energy
Q12. Which is correct about half-life?
🔵 (A) For zero-order, t₁/₂ ∝ 1/[R]₀
🟢 (B) For first-order, t₁/₂ is constant
🟠 (C) For second-order, t₁/₂ ∝ [R]₀
🔴 (D) For first-order, t₁/₂ ∝ [R]₀
Answer: (B) For first-order, t₁/₂ is constant
Q13. The activation energy is measured from:
🔵 (A) Reactants to products
🟢 (B) Reactants to transition state
🟠 (C) Products to reactants
🔴 (D) Products to transition state
Answer: (B) Reactants to transition state
Q14. A straight line graph of [R] vs t indicates:
🔵 (A) Zero-order
🟢 (B) First-order
🟠 (C) Second-order
🔴 (D) Third-order
Answer: (A) Zero-order
Q15. The frequency factor A in Arrhenius equation is related to:
🔵 (A) ΔH
🟢 (B) Collision orientation and frequency
🟠 (C) Temperature
🔴 (D) Catalyst
Answer: (B) Collision orientation and frequency
Q16. The effect of temperature on k is explained by:
🔵 (A) Transition state theory
🟢 (B) Collision theory
🟠 (C) Arrhenius equation
🔴 (D) All of these
Answer: (D) All of these
Q17. If a reaction is zero-order in [A], doubling [A] will:
🔵 (A) Double rate
🟢 (B) Halve rate
🟠 (C) Not affect rate
🔴 (D) Increase rate four times
Answer: (C) Not affect rate
Q18. Which of the following has units of L mol⁻¹ s⁻¹?
🔵 (A) Zero-order
🟢 (B) First-order
🟠 (C) Second-order
🔴 (D) Third-order
Answer: (C) Second-order
Q19. The Arrhenius plot of ln k vs 1/T gives slope = −5000. Eₐ = ? (R = 8.314 J/mol·K)
🔵 (A) 16.6 kJ/mol
🟢 (B) 41.6 kJ/mol
🟠 (C) 8.3 kJ/mol
🔴 (D) 25.0 kJ/mol
Answer: (B) 41.6 kJ/mol
Q20. For a first-order reaction, if 75% is completed in 138.6 s, the half-life is:
🔵 (A) 46.2 s
🟢 (B) 69.3 s
🟠 (C) 92.4 s
🔴 (D) 138.6 s
Answer: (B) 69.3 s
🟩 Q21–Q40 (JEE Main Level)
Q21. A reaction is 1st order in A and 2nd order in B. If [A] is doubled and [B] halved, rate changes by:
🔵 (A) 1/2
🟢 (B) 2
🟠 (C) 1
🔴 (D) 4
Answer: (A) 1/2
Q22. The decomposition of N₂O₅ is studied at 300 K. If ln[N₂O₅] vs t gives slope −5 × 10⁻⁴, k = ?
🔵 (A) 5 × 10⁻⁴ s⁻¹
🟢 (B) 1.15 × 10⁻³ s⁻¹
🟠 (C) 0.5 × 10⁻³ s⁻¹
🔴 (D) 2.3 × 10⁻⁴ s⁻¹
Answer: (A) 5 × 10⁻⁴ s⁻¹
Q23. The half-life of a second-order reaction with [A]₀ = 0.1 M and k = 0.5 M⁻¹s⁻¹ is:
🔵 (A) 20 s
🟢 (B) 2 s
🟠 (C) 10 s
🔴 (D) 200 s
Answer: (A) 20 s
Q24. A reaction follows first-order kinetics with t₁/₂ = 50 min. Time required for 75% completion is:
🔵 (A) 25 min
🟢 (B) 50 min
🟠 (C) 100 min
🔴 (D) 150 min
Answer: (C) 100 min
Q25. For the reaction A + B → products, doubling [A] doubles rate, while doubling [B] quadruples rate. Rate law is:
🔵 (A) k[A][B]
🟢 (B) k[A][B]²
🟠 (C) k[A]²[B]
🔴 (D) k[A]²[B]²
Answer: (B) k[A][B]²
Q26. The rate constant k has same units as rate when order is:
🔵 (A) Zero
🟢 (B) First
🟠 (C) Second
🔴 (D) Third
Answer: (A) Zero
Q27. Which of the following is true for molecularity?
🔵 (A) It is always equal to order
🟢 (B) It can be fractional
🟠 (C) It is a theoretical concept for elementary steps
🔴 (D) It is determined experimentally
Answer: (C) It is a theoretical concept for elementary steps
Q28. If log k vs 1/T is plotted, intercept gives:
🔵 (A) −Eₐ/R
🟢 (B) ln A
🟠 (C) k
🔴 (D) A
Answer: (B) ln A
Q29. For the decomposition 2N₂O₅ → 4NO₂ + O₂, rate law is first order. If pressure increases by ΔP in time t, rate constant is:
🔵 (A) k = (2.303/t) log(P∞/Pt)
🟢 (B) k = (2.303/t) log(ΔP∞/ΔPt)
🟠 (C) k = (2.303/t) log(P∞ − P₀)/(P∞ − Pt)
🔴 (D) k = 2.303(P∞ − P₀)/t
Answer: (C) k = (2.303/t) log(P∞ − P₀)/(P∞ − Pt)
Q30. Which is correct for Arrhenius equation?
🔵 (A) ln k vs 1/T is linear
🟢 (B) Activation energy can be negative
🟠 (C) A depends on [R]₀
🔴 (D) Slope = +Eₐ/R
Answer: (A) ln k vs 1/T is linear
Q31. For a first-order reaction, t₉₀% = ?
🔵 (A) 2.303/k
🟢 (B) 0.693/k
🟠 (C) 4.606/k
🔴 (D) 0.301/k
Answer: (A) 2.303/k
Q32. If Eₐ = 60 kJ/mol, R = 8.314 J/mol·K, slope of ln k vs 1/T is:
🔵 (A) −7220
🟢 (B) −4800
🟠 (C) −6000
🔴 (D) −8000
Answer: (A) −7220
Q33. In decomposition of A, rate constant doubles when T increases from 300 K to 310 K. The temperature coefficient is:
🔵 (A) 1
🟢 (B) 2
🟠 (C) 4
🔴 (D) 0.5
Answer: (B) 2
Q34. Half-life of ¹⁴C is 5730 years. Age of sample with 1/16th activity = ?
🔵 (A) 22920 yr
🟢 (B) 14325 yr
🟠 (C) 5730 yr
🔴 (D) 11460 yr
Answer: (A) 22920 yr
Q35. Which of the following is NOT true?
🔵 (A) Rate constant increases with temperature
🟢 (B) Catalyst changes equilibrium constant
🟠 (C) Order is experimentally determined
🔴 (D) Molecularity is an integer for elementary steps
Answer: (B) Catalyst changes equilibrium constant
Q36. If t₁/₂ = 10 min at 27 °C, and 20 min at 17 °C, approximate Eₐ is:
🔵 (A) 54 kJ/mol
🟢 (B) 108 kJ/mol
🟠 (C) 27 kJ/mol
🔴 (D) 10 kJ/mol
Answer: (A) 54 kJ/mol
Q37. Reaction A + B → products is first order in each. If [A] doubles, [B] halves, rate change = ?
🔵 (A) Remains same
🟢 (B) Doubles
🟠 (C) Halves
🔴 (D) Quadruples
Answer: (A) Remains same
Q38. For which order is half-life independent of [R]₀?
🔵 (A) Zero
🟢 (B) First
🟠 (C) Second
🔴 (D) Third
Answer: (B) First
Q39. A reaction is first order in A and overall order = 2. Rate law is:
🔵 (A) k[A]
🟢 (B) k[A][B]
🟠 (C) k[A][B]²
🔴 (D) k[A]²
Answer: (B) k[A][B]
Q40. Units of rate constant for third-order reaction are:
🔵 (A) mol² L⁻² s⁻¹
🟢 (B) L² mol⁻² s⁻¹
🟠 (C) s⁻¹
🔴 (D) L mol⁻¹ s⁻¹
Answer: (B) L² mol⁻² s⁻¹
🟪 Q41–Q50 (JEE Advanced Level)
Q41. The decomposition of X follows first-order kinetics. After 60 min, 25% decomposes. Calculate t₁/₂.
🔵 (A) 138.6 min
🟢 (B) 120 min
🟠 (C) 90 min
🔴 (D) 69.3 min
Answer: (D) 69.3 min
Q42. For reaction A → products, rate constant doubles when T increases from 300 K to 310 K. Find Eₐ.
🔵 (A) 52 kJ/mol
🟢 (B) 26 kJ/mol
🟠 (C) 104 kJ/mol
🔴 (D) 10 kJ/mol
Answer: (A) 52 kJ/mol
Q43. A reaction is second order with respect to A. If [A] decreases from 0.5 to 0.25 M in 20 min, k = ?
🔵 (A) 0.1 M⁻¹ min⁻¹
🟢 (B) 0.2 M⁻¹ min⁻¹
🟠 (C) 0.05 M⁻¹ min⁻¹
🔴 (D) 0.4 M⁻¹ min⁻¹
Answer: (A) 0.1 M⁻¹ min⁻¹
Q44. A first-order reaction has k = 2.303 × 10⁻² s⁻¹. Find time for 90% completion.
🔵 (A) 100 s
🟢 (B) 200 s
🟠 (C) 300 s
🔴 (D) 400 s
Answer: (A) 100 s
Q45. The ratio of slopes of plots of ln[R] vs t for two first-order reactions with k₁ and k₂ is:
🔵 (A) k₁/k₂
🟢 (B) k₂/k₁
🟠 (C) −k₁/−k₂
🔴 (D) Both (A) and (C)
Answer: (D) Both (A) and (C)
Q46. In enzyme catalysis, rate initially increases with [S], then becomes constant. Explanation?
🔵 (A) Enzyme decomposes
🟢 (B) Enzyme sites saturated
🟠 (C) Product inhibits
🔴 (D) Temperature decreases
Answer: (B) Enzyme sites saturated
Q47. A first-order reaction is 20% complete in 10 min. Time for 60% completion = ?
🔵 (A) 30 min
🟢 (B) 40 min
🟠 (C) 20 min
🔴 (D) 50 min
Answer: (A) 30 min
Q48. For a gaseous reaction, rate = kP. If pressure increases from 0.1 to 0.4 atm in 50 s, calculate k.
🔵 (A) 0.0139 s⁻¹
🟢 (B) 0.0278 s⁻¹
🟠 (C) 0.0556 s⁻¹
🔴 (D) 0.0069 s⁻¹
Answer: (B) 0.0278 s⁻¹
Q49. Temperature dependence of rate constant is given by ln k vs 1/T plot. If slope = −10⁴, activation energy is:
🔵 (A) 83.1 kJ/mol
🟢 (B) 41.6 kJ/mol
🟠 (C) 100 kJ/mol
🔴 (D) 25.0 kJ/mol
Answer: (A) 83.1 kJ/mol
Q50. A first-order reaction has rate constant k = 2.0 × 10⁻² s⁻¹. Time required for 90% completion is:
🔵 (A) 230 s
🟢 (B) 115 s
🟠 (C) 346 s
🔴 (D) 460 s
Answer: (A) 230 s
————————————————————————————————————————————————————————————————————————————
MIND MAPS
————————————————————————————————————————————————————————————————————————————
