Class 10: Science (In English) – Lesson 4. Carbon and its Compounds
EXPLANATION & SUMMARY
🌿 (Part 1) Explanation
🔵 Introduction
➡️ Carbon is a versatile element that forms the basis of all living and many non-living substances.
➡️ It is unique due to its ability to form a large number of compounds—organic compounds—due to catenation and tetravalency.
💡 Catenation: The ability of carbon to form long chains or rings with other carbon atoms through covalent bonds.
💡 Tetravalency: Carbon has four valence electrons and can form four covalent bonds with other atoms like hydrogen, oxygen, nitrogen, or other carbon atoms.
⚛️ Bonding in Carbon — Covalent Bonds
🔵 Covalent Bond:
➡️ It is formed by the mutual sharing of electron pairs between atoms.
➡️ Carbon shares electrons to complete its octet because it cannot easily lose or gain four electrons.
Examples:
✔️ In methane (CH₄), carbon shares one electron with each hydrogen atom → four covalent bonds form.

✔️ In oxygen (O₂), two atoms share two pairs of electrons → a double bond.

✔️ In nitrogen (N₂), three pairs of electrons are shared → a triple bond.

✏️ Note: Covalent compounds have low melting and boiling points and do not conduct electricity because they do not have free ions.
💡 Versatile Nature of Carbon
🟢 1. Catenation:
➡️ Carbon can link with other carbon atoms through single, double, or triple bonds to form long chains, branched structures, or rings.
➡️ Example: Methane (CH₄) → single bond, Ethene (C₂H₄) → double bond, Ethyne (C₂H₂) → triple bond.
🟡 2. Tetravalency:
➡️ Carbon’s four valence electrons allow it to bond with a wide range of elements such as H, O, N, Cl, S, etc.
🔴 3. Formation of Multiple Compounds:
➡️ Carbon forms millions of compounds — hydrocarbons, alcohols, acids, ketones, etc.
💡 Organic chemistry is the study of carbon compounds.
⚙️ Hydrocarbons
💡 Hydrocarbons are compounds made up of only carbon and hydrogen.
🔵 Types of Hydrocarbons:
1️⃣ Saturated Hydrocarbons (Alkanes):
➡️ Have only single covalent bonds.
➡️ General formula: CₙH₂ₙ₊₂
➡️ Example: Methane (CH₄), Ethane (C₂H₆).
2️⃣ Unsaturated Hydrocarbons:
➡️ Contain one or more double or triple bonds.
➡️ Alkenes (C=C) — e.g., Ethene (C₂H₄)
➡️ Alkynes (C≡C) — e.g., Ethyne (C₂H₂).
✏️ Note: Unsaturated hydrocarbons decolourise bromine water (used as a test for unsaturation).
🧪 Functional Groups in Carbon Compounds
💡 Functional group — an atom or group of atoms that determines the chemical properties of an organic compound.
Examples:
➡️ Alcohol group (–OH) → Ethanol (C₂H₅OH)
➡️ Aldehyde group (–CHO) → Ethanal (CH₃CHO)
➡️ Carboxylic acid group (–COOH) → Ethanoic acid (CH₃COOH)
➡️ Ketone group (–CO–) → Propanone (CH₃COCH₃)
💡 The presence of these groups changes the reactivity and properties of the compound.
🔬 Homologous Series
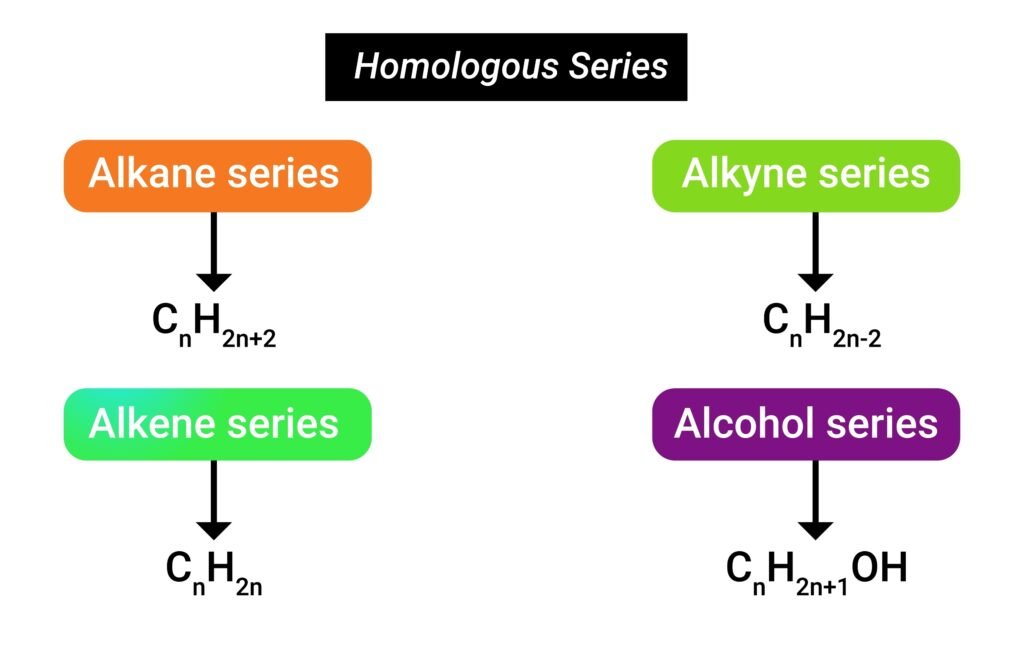
➡️ A homologous series is a family of organic compounds having the same functional group and general formula.
✔️ Example: Alkanes: CH₄, C₂H₆, C₃H₈, C₄H₁₀ … (difference of CH₂ group).
✔️ Each successive member differs by –CH₂– and shows similar chemical properties.
💡 Physical properties (like melting/boiling point) gradually increase with molecular mass.
🔥 Chemical Properties of Carbon Compounds
🟢 1. Combustion:
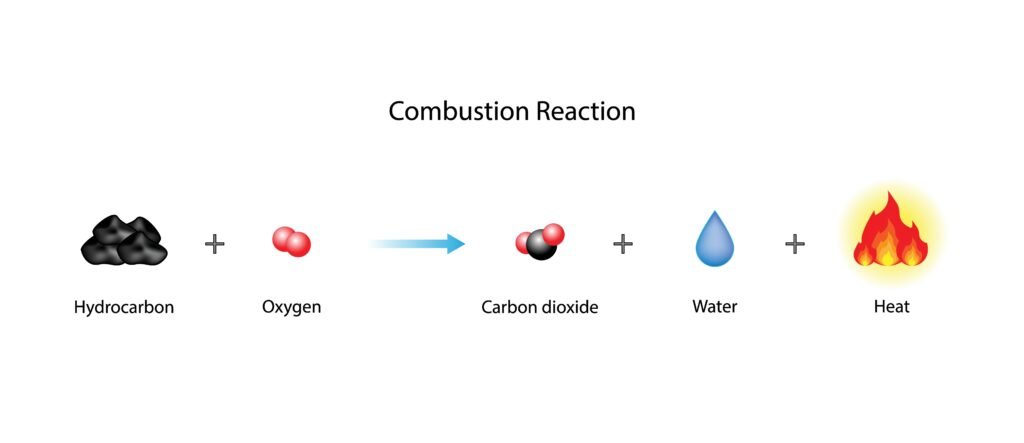
➡️ Carbon compounds burn in air (O₂) to produce CO₂, water, and heat.
➡️ CH₄ + 2O₂ → CO₂ + 2H₂O + Energy
💡 Used as fuels (LPG, CNG, petrol).
🔴 2. Oxidation:
➡️ Alcohols can be oxidised to acids using oxidising agents like alkaline KMnO₄ or acidified K₂Cr₂O₇.
➡️ Example: Ethanol → Ethanoic acid.
🟡 3. Addition Reaction:
➡️ Unsaturated hydrocarbons (C=C or C≡C) add hydrogen in the presence of a catalyst (Ni or Pt).
➡️ Example: C₂H₄ + H₂ → C₂H₆.
🔵 4. Substitution Reaction:
➡️ Occurs in saturated hydrocarbons.
➡️ Example: CH₄ + Cl₂ → CH₃Cl + HCl (in presence of sunlight).

⚡ Ethanol and Ethanoic Acid (Important Compounds)
🔵 Ethanol (C₂H₅OH)
✔️ Colourless liquid, soluble in water.
✔️ Commonly known as alcohol.
✔️ Used in medicines, fuels, and beverages.
💡 Reacts with sodium to form sodium ethoxide and hydrogen gas:
➡️ 2C₂H₅OH + 2Na → 2C₂H₅ONa + H₂↑
🟢 Ethanoic Acid (CH₃COOH)
✔️ Known as acetic acid.
✔️ Gives sour taste to vinegar (~5–8% solution).
✔️ Reacts with ethanol to form an ester (fruity-smelling compound):
➡️ CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
💡 Reaction is called esterification.
🧠 Cleansing Action of Soaps and Detergents
💡 Soaps are sodium or potassium salts of long-chain fatty acids.
➡️ Example: Sodium stearate (C₁₇H₃₅COONa).
🔵 How Soap Works:
➡️ Soap molecules have two parts —
• Hydrophobic tail (repels water, attracts oil/grease).
• Hydrophilic head (attracts water).
➡️ When soap is used, tails attach to oil droplets while heads remain in water forming micelles.
➡️ On rinsing, these micelles get washed away, removing dirt.
✏️ Note: Soaps are ineffective in hard water (form scum), but detergents work in both soft and hard water.
💡 Environmental Impact
➡️ Carbon compounds like fuels release CO₂ upon burning, contributing to global warming.
➡️ Ethanol blending with petrol reduces pollution.
➡️ Biodegradable detergents help protect water bodies.
📖 (Part 2) Summary
🔵 Carbon shows tetravalency and catenation forming millions of compounds.
🟢 Hydrocarbons are classified as alkanes, alkenes, and alkynes.
🔴 Functional groups define chemical behaviour of organic compounds.
🟡 Homologous series show gradation in properties.
✔️ Ethanol and ethanoic acid are important carbon compounds.
💡 Soaps and detergents remove dirt through micelle formation.
📝 (Part 3) Quick Recap
1️⃣ Carbon forms covalent bonds and shows catenation.
2️⃣ Functional groups modify chemical behaviour.
3️⃣ Ethanol forms sodium ethoxide; ethanoic acid forms esters.
4️⃣ Combustion, oxidation, addition, and substitution are key reactions.
5️⃣ Soaps act through micelles; detergents work even in hard water.
————————————————————————————————————————————————————————————————————————————
QUESTIONS FROM TEXTBOOK
🔵 Question 1:
Ethane, with the molecular formula C₂H₆, has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
✔️ Answer: (c) 8 covalent bonds
💡 Ethane (C₂H₆) has 6 C–H single bonds and 1 C–C bond, giving a total of 7, but since each bond is shared by two atoms, there are 8 shared pairs of electrons forming 8 covalent bonds.
🟢 Question 2:
Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
✔️ Answer: (c) ketone
💡 Butanone (CH₃COCH₂CH₃) contains the functional group >C=O (carbonyl group) present within the carbon chain — characteristic of ketones.
🔴 Question 3:
While cooking, if the bottom of the vessel is getting blackened on the outside, it means that —
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
✔️ Answer: (b) the fuel is not burning completely.
💡 Incomplete combustion produces carbon particles (soot) that blacken the vessel. Complete combustion produces only CO₂ and H₂O.
🟡 Question 4:
Explain the nature of the covalent bond using the bond formation in CH₃Cl.
✔️ Answer:
➡️ In CH₃Cl (chloromethane), carbon shares four electrons with three hydrogen atoms and one chlorine atom.
➡️ Each C–H and C–Cl bond is formed by mutual sharing of electrons.
➡️ These are single covalent bonds.
💡 No ions are formed, hence CH₃Cl is a covalent compound with low melting point and poor conductivity.
🔵 Question 5:
Draw the electron dot structures for:
(a) Ethanoic acid (CH₃COOH)
(b) H₂S
(c) Propanone (CH₃COCH₃)
(d) F₂
✔️ Answer:
(a) CH₃COOH → Each O forms 2 bonds, C forms 4 bonds, and H forms 1 bond.
(b) H₂S → S shares one pair with each H atom.
(c) CH₃COCH₃ → Central carbon (C=O) double bond with oxygen; each side carbon single-bonded to three hydrogens.
(d) F₂ → Each fluorine shares one pair of electrons to form a single F–F bond.
💡 All these compounds show covalent bonding through electron sharing.
🟢 Question 6:
What is a homologous series? Explain with an example.
✔️ Answer:
➡️ A homologous series is a group of organic compounds having the same functional group and general formula, with each member differing by a –CH₂– unit.
➡️ Example: Alkanes — CH₄, C₂H₆, C₃H₈, C₄H₁₀, etc.
💡 Members show similar chemical properties and a gradual change in physical properties.
🔴 Question 7:
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
✔️ Answer:
Physical:
➡️ Ethanol is neutral and has a pleasant smell.
➡️ Ethanoic acid has a sour taste and gives vinegar smell.
Chemical:
➡️ Ethanol reacts with sodium to form sodium ethoxide and hydrogen gas.
➡️ Ethanoic acid reacts with sodium carbonate to liberate CO₂ gas.
🟡 Question 8:
Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
✔️ Answer:
➡️ Soap molecules have hydrophobic tails and hydrophilic heads.
➡️ In water, the hydrophobic tails surround oil droplets, forming micelles.
➡️ In ethanol, micelles are not formed because ethanol is not sufficiently polar to form such structures.
🔵 Question 9:
Why are carbon and its compounds used as fuels for most applications?
✔️ Answer:
➡️ Carbon compounds such as methane, LPG, petrol, etc., have high calorific values.
➡️ They burn easily in air with sufficient oxygen, releasing large amounts of energy.
💡 Complete combustion produces clean energy in the form of CO₂ and H₂O.
🟢 Question 10:
Explain the formation of scum when hard water is treated with soap.
✔️ Answer:
➡️ Hard water contains calcium (Ca²⁺) and magnesium (Mg²⁺) ions.
➡️ These react with the sodium salts of fatty acids (soap) to form insoluble precipitates — scum.
💡 This reduces soap efficiency.
🔴 Question 11:
What change will you observe if you test soap with litmus paper (red and blue)?
✔️ Answer:
➡️ Soaps are basic in nature.
➡️ Red litmus turns blue; blue litmus remains unchanged.
🟡 Question 12:
What is hydrogenation? What is its industrial application?
✔️ Answer:
➡️ Hydrogenation is the process of adding hydrogen to unsaturated hydrocarbons (double/triple bonds) in the presence of a catalyst (Ni or Pt).
➡️ Example: C₂H₄ + H₂ → C₂H₆
💡 Used in industries to convert vegetable oils into solid fats like ghee and margarine.
🔵 Question 13:
Which of the following hydrocarbons undergo addition reactions:
C₂H₆, C₃H₈, C₂H₂, and CH₄?
✔️ Answer: C₂H₂ (Ethyne)
💡 Only unsaturated hydrocarbons (those with double or triple bonds) undergo addition reactions.
🟢 Question 14:
Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
✔️ Answer:
➡️ Bromine water test: Unsaturated hydrocarbons decolourise bromine water, while saturated hydrocarbons do not.
🔴 Question 15:
Explain the mechanism of the cleaning action of soaps.
✔️ Answer:
➡️ Soap molecules have two parts — hydrophilic (water-loving head) and hydrophobic (oil-attracting tail).
➡️ The tails embed into greasy dirt, while heads remain in water forming micelles.
➡️ Upon rinsing, the micelles containing dirt particles are washed away, cleaning the surface.
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
[CBSE MODEL QUESTION PAPER]
ESPECIALLY MADE FROM THIS LESSON ONLY
⚙️ Section A: Q1–6 (1 Mark Each – MCQ Type)
🔵 Question 1: Which of the following statements about carbon is correct?
🔵 (A) It has 3 valence electrons.
🟢 (B) It forms ionic bonds easily.
🔴 (C) It forms covalent bonds.
🟡 (D) It is a metal.
✔️ Answer: (C) It forms covalent bonds.
💡 Carbon shares its four valence electrons with other atoms to form covalent compounds.
🔵 Question 2: The ability of carbon to form long chains and rings with other carbon atoms is called —
🔵 (A) Valency
🟢 (B) Catenation
🔴 (C) Tetravalency
🟡 (D) Isomerism
✔️ Answer: (B) Catenation
💡 Catenation allows carbon atoms to link with one another forming long stable chains.
🔵 Question 3: Which of the following is a saturated hydrocarbon?
🔵 (A) Ethene
🟢 (B) Ethyne
🔴 (C) Methane
🟡 (D) Benzene
✔️ Answer: (C) Methane
💡 Saturated hydrocarbons have only single covalent bonds.
🔵 Question 4: Which of the following functional groups is present in alcohols?
🔵 (A) –CHO
🟢 (B) –COOH
🔴 (C) –OH
🟡 (D) –CO–
✔️ Answer: (C) –OH
💡 The hydroxyl (–OH) group represents alcohols.
🔵 Question 5: Which compound does not decolourise bromine water?
🔵 (A) Ethane
🟢 (B) Ethene
🔴 (C) Ethyne
🟡 (D) Propene
✔️ Answer: (A) Ethane
💡 Only unsaturated hydrocarbons decolourise bromine water.
🔵 Question 6: Which statement about covalent compounds is true?
🔵 (A) They conduct electricity in solution.
🟢 (B) They have high melting points.
🔴 (C) They are usually soluble in water.
🟡 (D) They have low melting and boiling points.
✔️ Answer: (D) They have low melting and boiling points.
⚡ Section B: Q7–12 (2 Marks Each – Short Answers)
🔵 Question 7: Why does carbon form covalent bonds?
✔️ Answer: Carbon has four valence electrons. It cannot gain or lose four electrons easily, so it shares electrons to form covalent bonds and complete its octet.
🟢 Question 8: What are hydrocarbons? Give one example.
✔️ Answer: Compounds made up of only carbon and hydrogen are called hydrocarbons.
➡️ Example: Methane (CH₄).
🔴 Question 9: What is a functional group? Give two examples.
✔️ Answer: A functional group is an atom or group of atoms that determines the chemical properties of a compound.
➡️ Examples: –OH (alcohol), –COOH (carboxylic acid).
🟡 Question 10: What are saturated and unsaturated hydrocarbons?
✔️ Answer:
➡️ Saturated hydrocarbons — contain only single bonds (e.g., Ethane C₂H₆).
➡️ Unsaturated hydrocarbons — contain double or triple bonds (e.g., Ethene C₂H₄, Ethyne C₂H₂).
🔵 Question 11: Why do covalent compounds have low melting and boiling points?
✔️ Answer: Covalent bonds involve sharing of electrons and have weak intermolecular forces, hence require less energy to break.
🟢 Question 12: Define homologous series and state its two characteristics.
✔️ Answer:
➡️ A homologous series is a group of organic compounds having the same functional group and general formula.
➡️ Characteristics:
1️⃣ Consecutive members differ by a –CH₂– unit.
2️⃣ They show similar chemical properties.
⚙️ Section C: Q13–22 (3 Marks Each – Mid-Length Answers)
🔵 Question 13: Write the structural formula of methane, ethane and ethene.
✔️ Answer:
➡️ Methane (CH₄): H–C–H (4 single bonds)
➡️ Ethane (C₂H₆): H₃C–CH₃
➡️ Ethene (C₂H₄): H₂C=CH₂
🟢 Question 14: Explain the versatile nature of carbon.
✔️ Answer:
1️⃣ Tetravalency — carbon forms four bonds with other atoms.
2️⃣ Catenation — ability to form long carbon chains.
3️⃣ Formation of multiple bonds — single, double, or triple.
4️⃣ Formation of various functional groups with O, H, N, Cl, etc.
🔴 Question 15: What happens when ethanol reacts with sodium metal?
✔️ Answer:
➡️ 2C₂H₅OH + 2Na → 2C₂H₅ONa + H₂↑
💡 Hydrogen gas is evolved, and sodium ethoxide is formed.
🟡 Question 16: Explain the process of esterification with an equation.
✔️ Answer:
➡️ Ethanoic acid reacts with ethanol in presence of conc. H₂SO₄ to form ester and water.
➡️ CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
💡 The ester has a fruity smell.
🔵 Question 17: What happens when ethanoic acid reacts with sodium carbonate?
✔️ Answer:
➡️ 2CH₃COOH + Na₂CO₃ → 2CH₃COONa + CO₂ + H₂O
💡 Effervescence of carbon dioxide gas confirms the reaction.
🟢 Question 18: List any three properties of ethanol.
✔️ Answer:
1️⃣ Colourless liquid, soluble in water.
2️⃣ Boiling point ≈ 78°C.
3️⃣ Reacts with sodium to liberate hydrogen gas.
🔴 Question 19: What is meant by oxidation of alcohols? Give example.
✔️ Answer: Oxidation is the process of adding oxygen or removing hydrogen.
➡️ Example: C₂H₅OH + [O] → CH₃COOH + H₂O (ethanol to ethanoic acid).
🟡 Question 20: Explain the term micelle.
✔️ Answer:
➡️ Soap molecules arrange themselves as spherical structures in water called micelles.
➡️ Their hydrophobic tails trap dirt while hydrophilic heads remain in water.
🔵 Question 21: Give reasons:
(a) Graphite conducts electricity but diamond does not.
(b) Soaps form scum in hard water.
✔️ Answer:
(a) Graphite has free electrons; diamond does not.
(b) Calcium and magnesium salts in hard water form insoluble scum with soap.
🟢 Question 22: Why are detergents better than soaps in hard water?
✔️ Answer:
➡️ Detergents do not react with calcium or magnesium ions; they form soluble salts.
➡️ Hence, they clean effectively even in hard water.
🧠 Section D: Q23–30 (4 Marks Each – Long & Case-Based)
🔵 Question 23: Describe the physical and chemical properties of covalent compounds.
✔️ Answer:
➡️ Physical: Low melting/boiling points, poor conductivity, often gaseous or liquid.
➡️ Chemical: React slowly, do not ionise in water.
💡 Example: CH₄, C₂H₅OH.
🟢 Question 24: Write four differences between soaps and detergents.
✔️ Answer:
1️⃣ Soap — sodium/potassium salt of fatty acid; Detergent — salt of sulphonic acid.
2️⃣ Soap ineffective in hard water; detergent effective.
3️⃣ Soap forms scum; detergent does not.
4️⃣ Detergents are stronger cleaning agents.
🔴 Question 25: Explain the reaction of unsaturated hydrocarbons with hydrogen.
✔️ Answer:
➡️ Addition reaction: H₂ adds to unsaturated hydrocarbons in presence of Ni or Pt catalyst.
➡️ C₂H₄ + H₂ → C₂H₆
💡 This process is called hydrogenation.
🟡 Question 26: What are esters? How are they formed and what happens when they are hydrolysed?
✔️ Answer:
➡️ Esters are fruity-smelling compounds formed by reaction of alcohol and acid.
➡️ CH₃COOH + C₂H₅OH → CH₃COOC₂H₅ + H₂O
➡️ On hydrolysis with base, they produce alcohol and salt of acid.
🔵 Question 27: Explain with examples the four types of reactions of carbon compounds.
✔️ Answer:
1️⃣ Combustion: CH₄ + 2O₂ → CO₂ + 2H₂O
2️⃣ Oxidation: C₂H₅OH + [O] → CH₃COOH + H₂O
3️⃣ Addition: C₂H₄ + H₂ → C₂H₆
4️⃣ Substitution: CH₄ + Cl₂ → CH₃Cl + HCl (in sunlight).
🟢 Question 28: Case study — Ramesh used soap in hard water and observed scum formation, while his friend used detergent and found better cleaning. Explain scientifically.
✔️ Answer:
➡️ Hard water contains Ca²⁺ and Mg²⁺ ions that form insoluble salts with soap (scum).
➡️ Detergents form soluble salts; thus, cleaning remains effective.
————————————————————————————————————————————————————————————————————————————
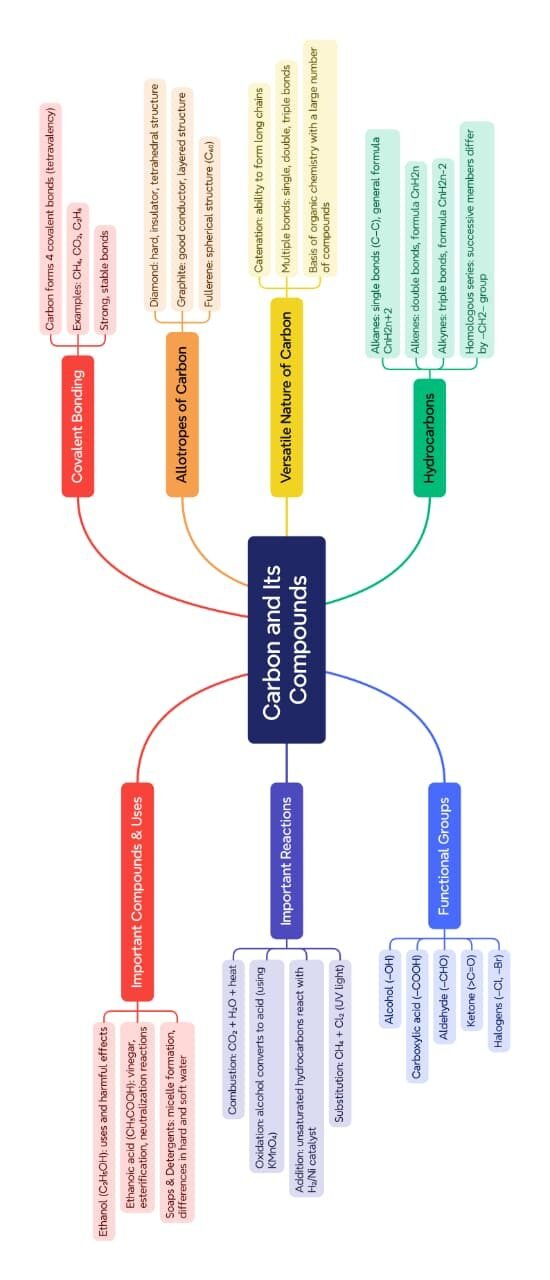
MIND MAPS
————————————————————————————————————————————————————————————————————————————
