Class : 9 – Science (English) : Lesson 4. Structure of the Atom
EXPLANATION & SUMMARY
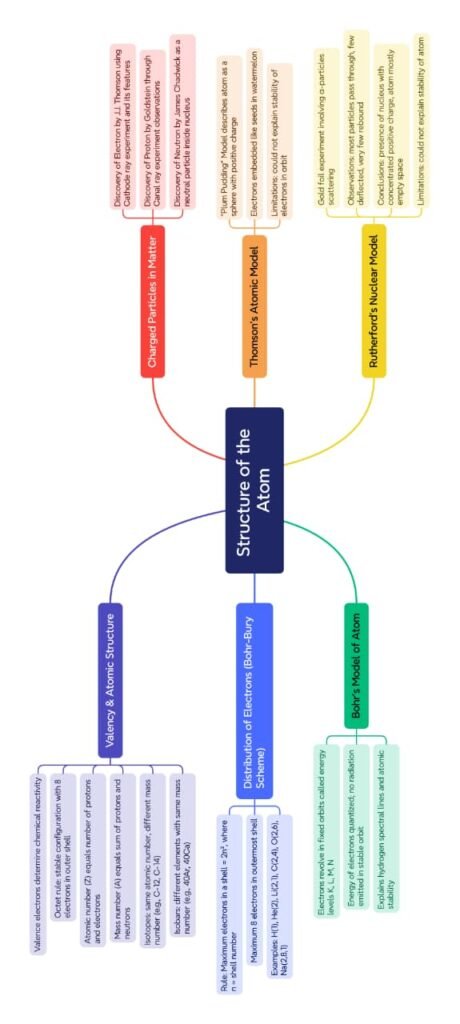
The chapter Structure of the Atom explores the internal structure of the atom and explains how subatomic particles like electrons, protons, and neutrons are arranged. This lesson builds upon the idea that atoms are not indivisible, as earlier believed in Dalton’s atomic theory, and introduces various models proposed by scientists to explain atomic structure.
Atoms consist of three fundamental particles:
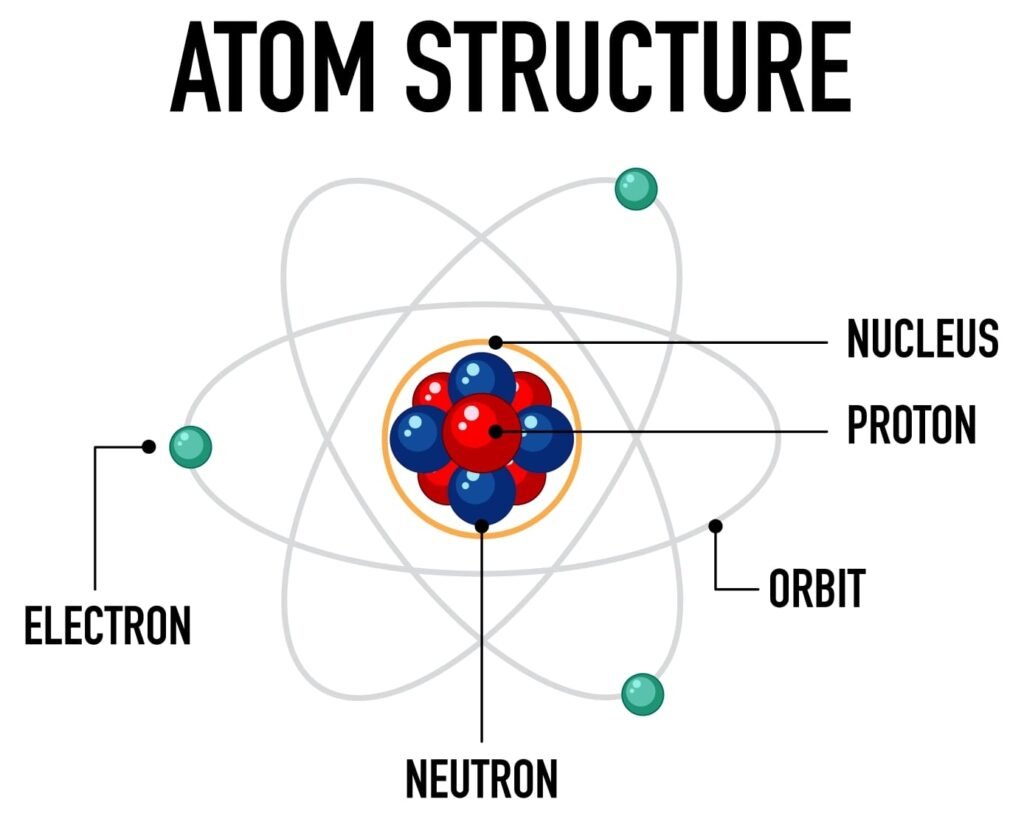
Electrons: Negatively charged particles discovered by J.J. Thomson using cathode ray experiments.
Protons: Positively charged particles discovered by Goldstein through anode ray experiments.
Neutrons: Neutral particles discovered later by James Chadwick.
The discovery of these particles led to the downfall of Dalton’s model. J.J. Thomson proposed the first model called the Plum Pudding Model, where he visualized the atom as a sphere of positive charge with negatively charged electrons embedded in it, like plums in a pudding. However, this model could not explain the results of later experiments.
The most significant experiment that changed our understanding of the atom was the Gold Foil Experiment conducted by Ernest Rutherford. He directed alpha particles at a thin gold foil and observed that most particles passed through, some were deflected at small angles, and a few rebounded. This led him to propose the Rutherford’s Nuclear Model of Atom, which stated:
Atoms have a small, dense, positively charged nucleus at the center.
Most of the atom is empty space.
Electrons revolve around the nucleus in circular orbits.
Despite its breakthrough, Rutherford’s model was incomplete because it couldn’t explain why electrons don’t spiral into the nucleus due to attraction.
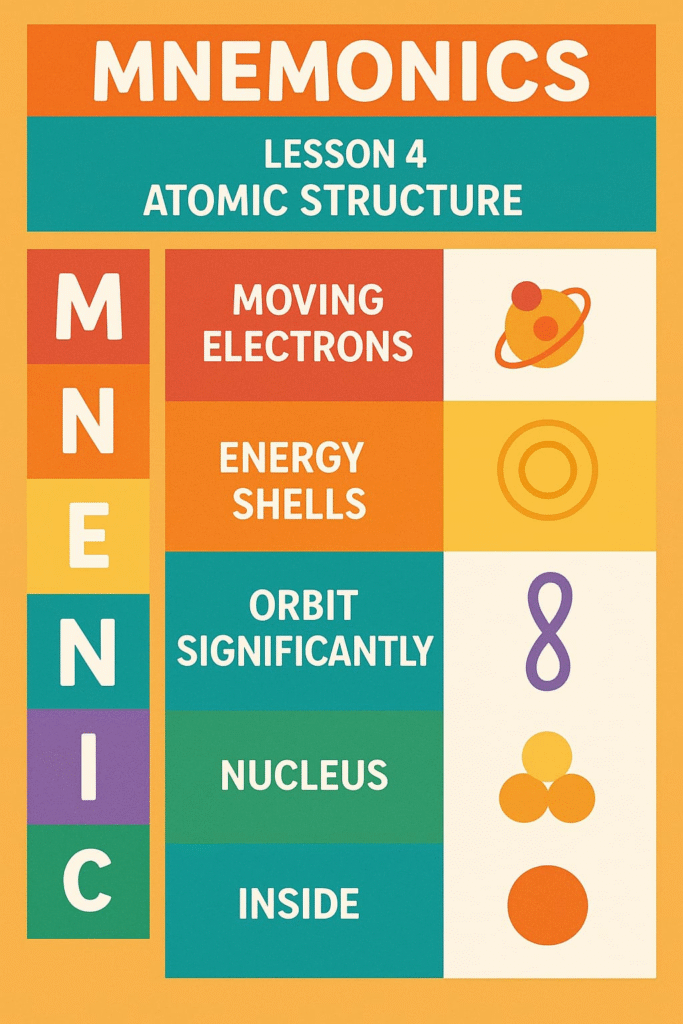
To resolve this, Niels Bohr proposed the Bohr Model, which refined Rutherford’s model. According to Bohr:
Electrons revolve in specific circular orbits called energy levels or shells, denoted as K, L, M, N, etc.
As long as an electron remains in a particular orbit, it does not lose energy.
Electrons can move between energy levels by absorbing or emitting fixed amounts of energy (quanta).
Bohr’s model successfully explained the stability of the atom and the line spectra of elements.
The chapter also introduces the concept of atomic number (Z) and mass number (A):
Atomic number (Z) is the number of protons in the nucleus. It is unique for each element.
Mass number (A) is the sum of protons and neutrons in the nucleus.
For example, carbon has 6 protons and 6 neutrons. So, its atomic number is 6 and mass number is 12.
The concept of isotopes and isobars is also discussed:
Isotopes are atoms of the same element with the same atomic number but different mass numbers (e.g., Hydrogen – ¹H, ²H, ³H).
Isobars are atoms with the same mass number but different atomic numbers (e.g., ⁴⁰Ca and ⁴⁰Ar).
————————————————————————————————————————————————————————————————————————————
TEXTBOOK QUESTIONS
🔵 Q1. Compare the properties of electrons, protons and neutrons.
🟢 Answer:
Property Electron (e⁻) Proton (p⁺) Neutron (n⁰)
Charge Negative (-1) Positive (+1) Neutral (0)
Mass 1/1836 u 1 u 1 u
Location Outside nucleus Inside nucleus Inside nucleus
Symbol e⁻ p⁺ n⁰
Relative Mass ~0 1 1
🔵 Q2. What are the limitations of J.J. Thomson’s model of the atom?
🟢 Answer:
✖ Thomson’s model (plum pudding model) could not explain:
➡ Stability of the atom
➡ Position of nucleus
➡ Observations from Rutherford’s experiment
➡ Distribution of positive charge
🔵 Q3. What are the limitations of Rutherford’s model of the atom?
🟢 Answer:
⚠ Rutherford’s model failed to explain:
✔ Why electrons don’t spiral into the nucleus due to attraction
✔ Stability of the atom
✔ Electron energy levels
✔ Spectrum of hydrogen atom
🔵 Q4. Describe Bohr’s model of the atom.
🟢 Answer:
💡 Niels Bohr proposed that:
✔ Electrons revolve in fixed circular orbits (shells).
✔ Each orbit has a fixed energy (energy levels).
✔ Electrons do not radiate energy while in orbit.
✔ Electrons absorb/emit energy only when jumping between levels.
🎯 Shells are denoted as K, L, M, N…
🔵 Q5. Compare all the proposed models of an atom given in this chapter.
🟢 Answer:
Feature Thomson Model Rutherford Model Bohr Model
Structure Positive sphere with electrons inside Dense nucleus with electrons revolving Electrons in fixed orbits
Nucleus Not present Present (small & dense) Present
Stability Unexplained Unstable (according to classical physics) Stable orbits explained
Energy Levels Not defined Not defined Clearly defined
🔵 Q6. Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
🟢 Answer:
📘 Rules:
1️⃣ Maximum electrons in a shell = 2n² (where n = shell number)
2️⃣ Shells are filled in order: K → L → M → N
3️⃣ Outer shell should have max 8 electrons (octet rule).
4️⃣ Electrons are filled to maintain stability and energy hierarchy.
🔵 Q7. Define valency by taking examples of silicon and oxygen.
🟢 Answer:
🧪 Valency is the combining capacity of an atom.
✔ Silicon (Atomic no. 14) – Configuration: 2, 8, 4 → Valency = 4
✔ Oxygen (Atomic no. 8) – Configuration: 2, 6 → Valency = 2
💡 Valency = number of electrons lost, gained, or shared to complete octet.
🔵 Q8. Explain with examples: (i) Atomic number, (ii) Mass number, (iii) Isotopes, and (iv) Isobars. Give any two uses of isotopes.
🟢 Answer:
(i) ➡ Atomic number (Z): Number of protons.
Example: Carbon (Z = 6)
(ii) ➡ Mass number (A): Total protons + neutrons.
Example: Carbon (A = 12)
(iii) ➡ Isotopes: Atoms with same atomic number but different mass numbers.
Example: ¹⁶O and ¹⁸O
(iv) ➡ Isobars: Atoms with same mass number but different atomic numbers.
Example: ⁴⁰Ar and ⁴⁰Ca
✅ Uses of Isotopes:
✔ Cobalt-60 in cancer treatment
✔ Iodine-131 to treat thyroid disorders
🔵 Q9. Na⁺ has completely filled K and L shells. Explain.
🟢 Answer:
➡ Atomic number of sodium = 11
Normal configuration = 2, 8, 1
After losing 1 electron (Na⁺), configuration = 2, 8
✔ K and L shells are completely filled.
🔵 Q10. If bromine atom is available in the form of, say, two isotopes:
₃₅Br⁷⁹ (49.7%) and ₃₅Br⁸¹ (50.3%), calculate the average atomic mass.
🟢 Answer:
Average mass = (79 × 49.7/100) + (81 × 50.3/100)
= 39.263 + 40.743
= 80.006 u
🔵 Q11. The average atomic mass of element X is 16.2 u.
What are the percentages of isotopes ₈X¹⁶ and ₈X¹⁸ in the sample?
🟢 Answer:
Let % of ₈X¹⁶ = x → ₈X¹⁸ = (100 – x)
So,
16x + 18(100 – x) = 16.2 × 100
Solving:
16x + 1800 – 18x = 1620
-2x = -180
x = 90%
So,
✔ ₈X¹⁶ = 90%, ₈X¹⁸ = 10%
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
1. The electron was discovered by:
A) Dalton
B) Rutherford
C) J.J. Thomson
D) Bohr
Answer: C) J.J. Thomson
2. In Rutherford’s gold foil experiment, most alpha particles:
A) Rebounded
B) Passed through the foil
C) Were absorbed
D) Got deflected at right angles
Answer: B) Passed through the foil
3. Neutrons were discovered by:
A) J.J. Thomson
B) Niels Bohr
C) James Chadwick
D) Ernest Rutherford
Answer: C) James Chadwick
4. The nucleus of an atom contains:
A) Only electrons
B) Protons and neutrons
C) Only protons
D) Electrons and protons
Answer: B) Protons and neutrons
5. Which of the following is an isotope of hydrogen?
A) O-16
B) H-1
C) He-4
D) C-14
Answer: B) H-1
Fill in the Blanks
1. The atomic number is equal to the number of _ in an atom.
Answer: protons
2. Electrons revolve around the nucleus in fixed paths called _.
Answer: shells or energy levels
3. The mass number of an atom is the sum of protons and _.
Answer: neutrons
4. The number of protons and electrons is equal in a _ atom.
Answer: neutral
5.The maximum number of electrons in the first shell (K-shell) is _.
Answer: 2
Very Short Answer Questions (1–2 lines)
1. What is the charge on a neutron?
Answer: A neutron has no charge; it is neutral.
2. Who proposed the planetary model of the atom?
Answer: Rutherford.
3. What is the symbol of the isotope of hydrogen with one neutron?
Answer: ²H or Deuterium.
4. What is the mass number of an atom having 11 protons and 12 neutrons?
Answer: 23
5. Which subatomic particle is negatively charged?
Answer: Electron
Short Answer Questions (30–50 words)
1. Define atomic number and mass number.
Answer:
Atomic number (Z) is the number of protons in the nucleus of an atom.
Mass number (A) is the total number of protons and neutrons in the nucleus of an atom.
2. What are isotopes? Give one example.
Answer: Isotopes are atoms of the same element with the same atomic number but different mass numbers.
Example: ¹H, ²H, and ³H are isotopes of hydrogen.
3. State two observations from Rutherford’s alpha particle experiment.
Answer:
Most alpha particles passed through the gold foil.
A few were deflected at small angles, and very few rebounded.
4. What is the valency of an element with atomic number 8?
Answer:
Oxygen (atomic number 8) has 6 electrons in its outer shell. Its valency is 2 (8 – 6 = 2).
5. What are isobars? Give an example.
Answer:
Isobars are atoms with the same mass number but different atomic numbers.
Example: ⁴⁰Ca and ⁴⁰Ar.
Detailed Answer Questions (80–120 words)
1. Describe Bohr’s model of the atom.
Answer:
Bohr proposed that electrons revolve around the nucleus in fixed circular paths called energy levels or shells (K, L, M, N). These shells have fixed energy. Electrons do not radiate energy while moving in these shells. However, electrons can jump from one shell to another by absorbing or releasing energy in discrete quantities (quanta). This model explained the stability of the atom and the discrete line spectra of elements. It improved upon Rutherford’s model by introducing energy levels and explaining why electrons don’t spiral into the nucleus.
2. Explain Rutherford’s alpha particle scattering experiment and its conclusions.
Answer:
Rutherford bombarded a thin gold foil with alpha particles. He observed that:
Most alpha particles passed straight through.
Some deflected at small angles.
Very few rebounded back.
From this, he concluded that:
Most of the atom is empty space.
A small, dense, positively charged nucleus is at the center.
Electrons revolve around the nucleus.
This experiment led to the nuclear model of the atom but couldn’t explain the stability of electron orbits, which was later addressed by Bohr.
————————————————————————————————————————————————————————————————————————————
MNEMONICS
————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN
————————————————————————————————————————————————————————————————————————————