Class : 9 – Science (English) : Lesson 3. Atoms and Molecules
EXPLANATION & SUMMARY
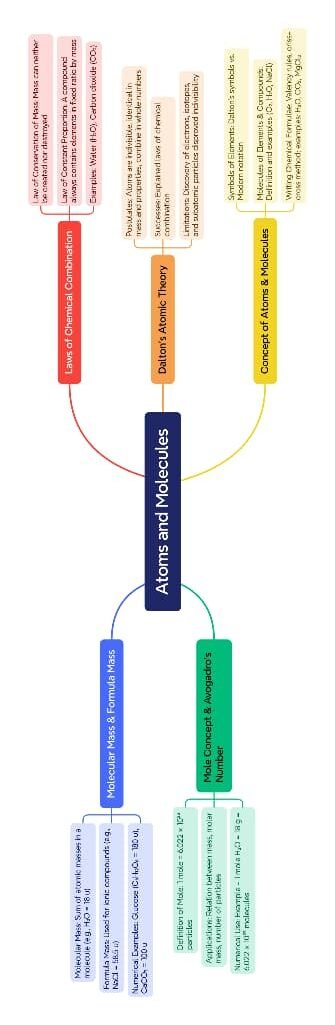
The chapter Atoms and Molecules from Class 9 NCERT Science builds a strong foundation in basic chemistry by exploring how matter is composed at the atomic and molecular level. It connects earlier concepts of matter with chemical combination and explains how atoms and molecules form the basis of all substances.
The lesson begins with the historical development of atomic theory. The idea that matter is made up of tiny indivisible particles dates back to ancient times, with thinkers like Maharishi Kanad in India and Democritus in Greece proposing early ideas about atoms. This was later formalized by John Dalton, who proposed the Dalton’s Atomic Theory. His key postulates include:
All matter is made of indivisible atoms.
Atoms of an element are identical in mass and properties.
Atoms combine in whole-number ratios to form compounds.
Atoms are neither created nor destroyed in chemical reactions.
The chapter then discusses the concept of atoms, the smallest unit of an element. Each atom is represented by a symbol, mostly based on English or Latin names (e.g., O for oxygen, Fe for iron from Latin “ferrum”). These symbols are universally accepted and form the language of chemistry.
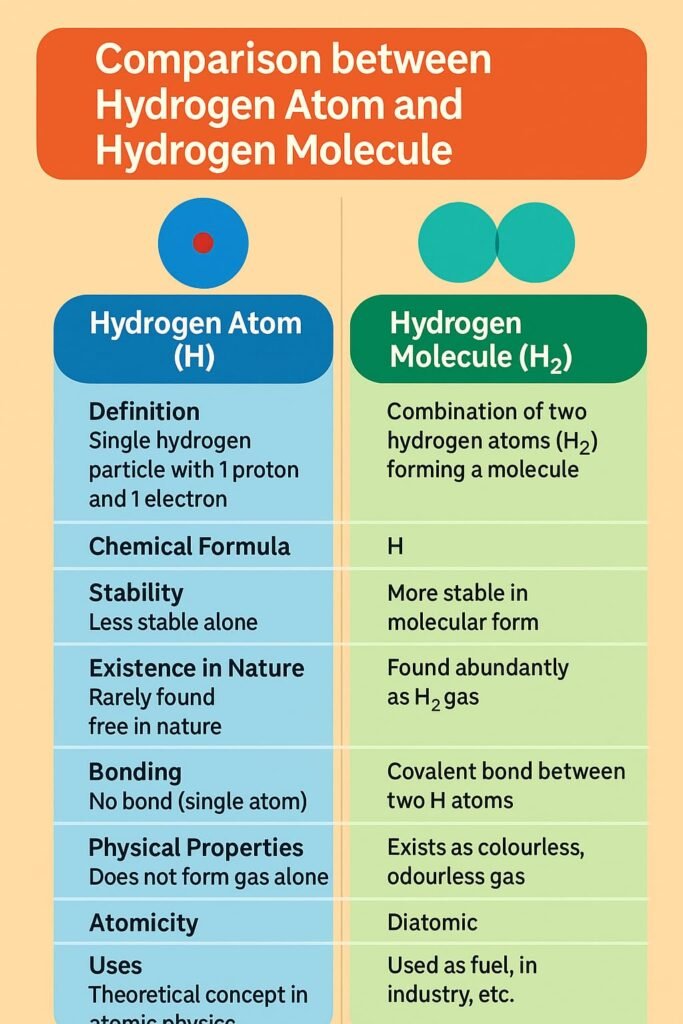
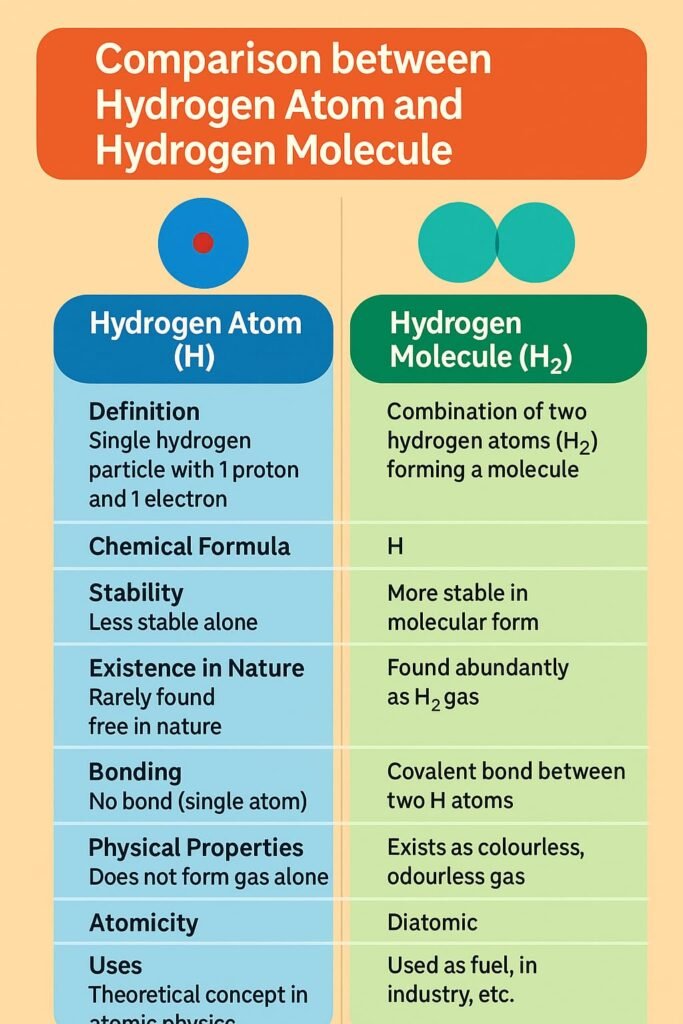
Atoms rarely exist alone in nature. They combine to form molecules, which are groups of two or more atoms bonded together. Molecules may consist of the same kind of atoms (e.g., O₂, N₂) or different kinds (e.g., H₂O, CO₂). The molecule of an element contains only one type of atom, while the molecule of a compound contains two or more different atoms chemically bonded.
The chapter introduces the concept of the atomic mass unit (amu), where 1 amu is defined as one-twelfth the mass of a carbon-12 atom. The molecular mass of a compound is calculated by adding the atomic masses of the atoms present in the molecule.
Next, the concept of ions is explained. Ions are charged particles formed when atoms gain or lose electrons. Cations are positively charged ions (e.g., Na⁺), and anions are negatively charged ions (e.g., Cl⁻). Compounds formed by the combination of cations and anions are called ionic compounds (e.g., NaCl).
A key part of the chapter is chemical formulae—representations of compounds using symbols and numerical subscripts. For example, H₂O means a molecule contains two hydrogen atoms and one oxygen atom. To write a chemical formula, one must know the valency (combining capacity) of each atom.
The chapter introduces the mole concept, which is essential for quantifying matter. One mole contains 6.022 × 10²³ particles (Avogadro’s number). The molar mass of a substance is the mass of one mole of its particles, numerically equal to its molecular mass in grams.
In conclusion, the lesson emphasizes that understanding atoms and molecules helps explain the behavior and interaction of matter. It prepares students for deeper chemical concepts, such as chemical reactions and equations, by giving them tools to visualize and calculate the smallest units of matter in physical and chemical processes.
———————————————————————————————————————————————————————————————————————————-
TEXTBOOK QUESTIONS
Question 1
A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer:
Given data:
Total mass of the compound = 0.24 g
Mass of boron in the compound = 0.096 g
Mass of oxygen in the compound = 0.144 g
The percentage composition formula is:
Percentage of an element = (Mass of element / Total mass of compound) × 100
Percentage of Boron:
Percentage of Boron = (0.096 g / 0.24 g) × 100 = 40%
Percentage of Oxygen:
Percentage of Oxygen = (0.144 g / 0.24 g) × 100 = 60%
Therefore, the compound contains 40% boron and 60% oxygen by weight.
Question 2
When 3.0 g of carbon is burnt in 8.00 g oxygen, 11.00 g of carbon dioxide is produced. What mass of carbon dioxide will be formed when 3.00 g of carbon is burnt in 50.00 g of oxygen? Which law of chemical combination will govern your answer?
Answer:
According to the given data:
3.0 g of carbon combines with 8.0 g of oxygen to produce 11.0 g of carbon dioxide
When 3.00 g of carbon is burnt in 50.00 g of oxygen, the reaction will still follow the Law of Definite Proportions (Law of Constant Proportions). This law states that in a pure chemical compound, elements are always present in definite proportions by mass.
Since carbon and oxygen always combine in a fixed ratio of 3:8 by mass, only 8.00 g of oxygen will be used to react with 3.00 g of carbon. The remaining 42.00 g of oxygen will remain unreacted.
Therefore, 11.00 g of carbon dioxide will be formed, and the Law of Definite Proportions governs this answer.
Question 3
What are polyatomic ions? Give examples.
Answer:
Polyatomic ions are ions that contain more than one atom. These ions consist of a group of atoms that carry a net positive or negative charge and behave as a single unit in chemical reactions.
The atoms in polyatomic ions can be of the same type or different types, and they are held together by covalent bonds. These ions carry a fixed charge and participate in ionic compound formation.
Examples of polyatomic ions:
Ammonium ion (NH₄⁺) – carries a +1 charge
Hydroxide ion (OH⁻) – carries a -1 charge
Sulfate ion (SO₄²⁻) – carries a -2 charge
Sulfite ion (SO₃²⁻) – carries a -2 charge
Carbonate ion (CO₃²⁻) – carries a -2 charge
Nitrate ion (NO₃⁻) – carries a -1 charge
Question 4
Write the chemical formulae of the following:
(a) Magnesium chloride
(b) Calcium oxide
(c) Copper nitrate
(d) Aluminium chloride
(e) Calcium carbonate
Answer:
The chemical formulae are determined by balancing the charges of the constituent ions:
(a) Magnesium chloride: MgCl₂
Mg²⁺ (magnesium ion) + Cl⁻ (chloride ion)
To balance charges: one Mg²⁺ requires two Cl⁻ ions
(b) Calcium oxide: CaO
Ca²⁺ (calcium ion) + O²⁻ (oxide ion)
Charges are already balanced in 1:1 ratio
(c) Copper nitrate: Cu(NO₃)₂
Cu²⁺ (copper ion) + NO₃⁻ (nitrate ion)
To balance charges: one Cu²⁺ requires two NO₃⁻ ions
(d) Aluminium chloride: AlCl₃
Al³⁺ (aluminium ion) + Cl⁻ (chloride ion)
To balance charges: one Al³⁺ requires three Cl⁻ ions
(e) Calcium carbonate: CaCO₃
Ca²⁺ (calcium ion) + CO₃²⁻ (carbonate ion)
Charges are already balanced in 1:1 ratio
Question 5
Give the names of the elements present in the following compounds:
(a) Quick lime
(b) Hydrogen bromide
(c) Baking powder
(d) Potassium sulphate
Answer:
(a) Quick lime (CaO):
Calcium and Oxygen
(b) Hydrogen bromide (HBr):
Hydrogen and Bromine
(c) Baking powder (NaHCO₃):
Sodium, Hydrogen, Carbon, and Oxygen
(d) Potassium sulphate (K₂SO₄):
Potassium, Sulfur, and Oxygen
Question 6
Calculate the molar mass of the following substances:
(a) Ethyne, C₂H₂
(b) Sulphur molecule, S₈
(c) Phosphorus molecule, P₄ (Atomic mass of phosphorus = 31)
(d) Hydrochloric acid, HCl
(e) Nitric acid, HNO₃
Answer:
The molar mass is calculated by adding the atomic masses of all atoms in the molecular formula:
(a) Ethyne (C₂H₂):
Molar mass = (2 × 12) + (2 × 1) = 24 + 2 = 26 u
(b) Sulphur molecule (S₈):
Molar mass = 8 × 32 = 256 u
(c) Phosphorus molecule (P₄):
Molar mass = 4 × 31 = 124 u
(d) Hydrochloric acid (HCl):
Molar mass = 1 + 35.5 = 36.5 u
(e) Nitric acid (HNO₃):
Molar mass = 1 + 14 + (3 × 16) = 1 + 14 + 48 = 63 u
————————————————————————————————————————————————————————————————————————————
OTHER IMPORTANT QUESTIONS FOR EXAMS
(MCQs)
1. The smallest particle of an element that can take part in a chemical reaction is:
A) Molecule
B) Ion
C) Atom
D) Compound
Answer: C) Atom
2. Which of the following is a triatomic molecule?
A) O₂
B) HCl
C) CO
D) O₃
Answer: D) O₃
3. The chemical formula of calcium chloride is:
A) CaCl
B) CaCl₂
C) Ca₂Cl
D) Ca₂Cl₂
Answer: B) CaCl₂
4. One mole of any substance contains:
A) 6.023 × 10¹⁹ atoms
B) 6.022 × 10²³ atoms
C) 3.03 × 10²⁰ atoms
D) 12.000 atoms
Answer: B) 6.022 × 10²³ atoms
5. Which of these is a cation?
A) Cl⁻
B) O²⁻
C) Na⁺
D) N³⁻
Answer: C) Na⁺
Fill in the Blanks
1. The atomicity of nitrogen is _.
Answer: 2
2. The formula of water is _.
Answer: H₂O
3. _ is the number of particles present in one mole of a substance.
Answer: Avogadro’s number
4. A molecule of an element contains _ types of atoms.
Answer: One
5. The molecular mass of CO₂ is _ u.
Answer: 44
Very Short Answer Questions (1–2 lines)
1. What is an ion?
Answer: An ion is a charged particle formed by the gain or loss of electrons by an atom.
2. What is the symbol of gold?
Answer: Au
3. Name one diatomic molecule.
Answer: Oxygen (O₂)
4. What is the valency of hydrogen?
Answer: 1
5. What is the chemical formula of magnesium oxide?
Answer: MgO
Short Answer Questions (30–50 words)
1. Define atomic mass unit.
Answer: One atomic mass unit (amu) is defined as one-twelfth the mass of a carbon-12 atom. It is used to express atomic and molecular masses.
2. Write the names of the elements present in the compound Na₂SO₄.
Answer: Sodium (Na), Sulphur (S), and Oxygen (O).
3. State two differences between molecules and compounds.
Answer:
Molecules can be of elements or compounds, but compounds are made of different elements only.
A molecule may contain similar atoms (O₂), but a compound contains different atoms (H₂O).
4. Define valency and give the valency of calcium.
Answer: Valency is the combining capacity of an atom. The valency of calcium is 2.
5. What do you understand by chemical formula?
Answer: A chemical formula is a symbolic representation of a compound showing the types and number of atoms of each element present in one molecule of the compound.
Detailed Answer Questions (80–120 words)
1. State the main postulates of Dalton’s Atomic Theory.
Answer:
Dalton’s Atomic Theory proposed the following:
All matter is made up of tiny indivisible particles called atoms.
Atoms of a given element are identical in mass and properties.
Atoms cannot be created, divided, or destroyed.
Atoms combine in simple whole-number ratios to form compounds.
In chemical reactions, atoms are rearranged but not transformed into other atoms.
This theory laid the foundation of modern chemistry by introducing the idea of atoms as the basic building blocks of matter.
2. Explain the mole concept. How is it useful in chemistry?
Answer:
The mole is a unit that measures the amount of substance. One mole contains 6.022 × 10²³ particles (Avogadro’s number), whether they are atoms, molecules, or ions.
For example, 1 mole of oxygen molecules (O₂) contains 6.022 × 10²³ O₂ molecules and weighs 32 g.
The mole concept allows chemists to count and relate the number of particles with mass in chemical equations. It simplifies calculations in stoichiometry, making it possible to measure reactants and products accurately in chemical reactions.
————————————————————————————————————————————————————————————————————————————
MNEMONICS

————————————————————————————————————————————————————————————————————————————
KNOWLEDGE WITH FUN

————————————————————————————————————————————————————————————————————————————